In comparison to advanced countries shortening their new drug approval period, the Korean drug regulator spends too much time to review drug approval, a group of foreign pharmaceutical companies operating in Korea said in a report.

The Korea Research-based Pharma Industry Association (KRPIA) initiated a study on the new drug approval period in Korea for 235 new drugs -- 166 chemical and 69 biological drugs -- that global pharmaceutical companies received approval in Korea for the past 10 years (2011-2020). The study was also participated by researchers from Sungkyunkwan University.
According to the study, the U.S. Food and Drug Administration, European Medicines Agency, and Japan’s Pharmaceuticals and Medical Devices Agency reduced the period for new drug approval and review by 9.6 days, 5.2 days, and 3 days, respectively, between 2011 and 2020.
In contrast, drug approval by Korea’s Ministry of Food and Drug Safety (MFDS) increased to 353 days in 2020 from 276 days in 2011.
The median approval and review period for chemical and biological drugs was 291 days and 353 days, and regression analysis showed that biological drugs took an average of 43.2 days longer during the 2011-2020 period.
Orphan drugs showed a faster approval and review period of 130.4 days than other new drugs, but in some cases, the period was similar or longer, despite the enactment of the Rare Disease Control Act in 2015 to promote orphan drug development and strengthen patient access.
The research team also analyzed the submission gap, which compares the date when a new drug was submitted for approval in the U.S. or Europe with that of Korea.
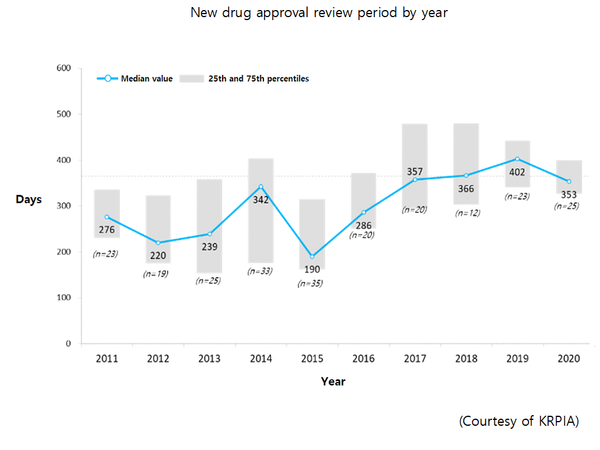
From 2011 to 2020, the approval gap, which compares the date a new drug was approved in the U.S. or EU and approved in Korea, showed that Korea approved new drugs 551 days after the drugs received approval in the U.S. or EU.
The study also found that the GMP review period during the approval and review process took longer on average than the safety and efficacy review or standard and test method review.
KRPIA emphasized that the availability and schedule of GMP on-site inspection and the corresponding review period had a great impact on the approval review period.
“Despite recent legislative initiatives, the delay in the new drug approval review timeline has steadily increased over 10 years in Korea,” the report concluded. “Careful enforcement of relevant laws and supplementary actions is required to increase new drug accessibility.”
Related articles
- Korea to exclude Australia from new drug pricing reference country list
- KRPIA raises concerns over Korea's plan to add Australia to drug pricing reference country list
- 31 multinational drug companies increased R&D in Korea by 20% in 2021: KRPIA
- Multinational pharmas' R&D in Korea up 14% in 2022: KRPIA

