KAIST said on Wednesday that its researchers identified a post-translational modification (PTM) code that can help to suppress cancer and other brain-related diseases.
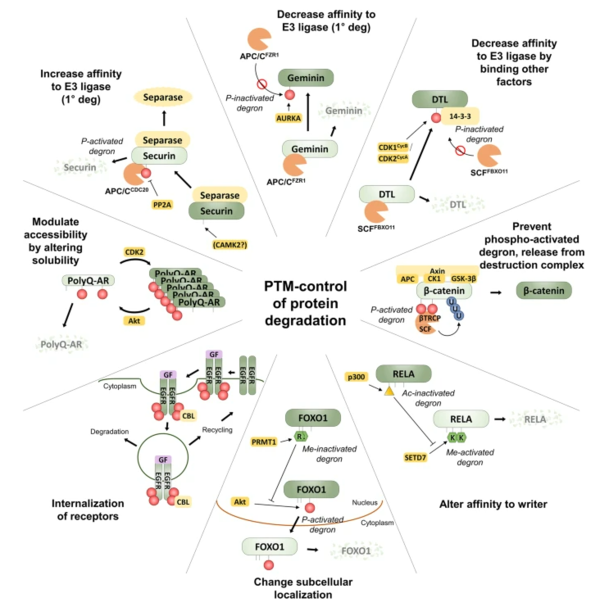
PTM refers to the translation process of the mRNA molecule into the final protein which affects its structure and efficacy of the protein. Accordingly, the regulation of this mechanism is responsible for the proper functioning of proteins. However, if not controlled, it can cause the accumulation of proteins.
The joint team of researchers from KAIST, the European Molecular Biology Laboratory, and Seoul National University investigated the regulation of protein stability by various PTMs.
The research team identified that the stability of these proteins is controlled by degron regions whereby PTM-activated degrons cause protein degradation and PTM-inactivated degrons forestall degradation and stabilize a protein.
In other words, the degron acts as a switch for disease progression or inhibition as a combination of amino acid sequences that can control protein levels.
As a result, the research team identified potential new drug targets to expand treatment options for patients where drug targets are currently lacking. Additionally, the team re-examined the role of enzymes in inducing or removing PTM in existing signaling schemes where the root cause of proteolysis and generation was unknown by diversifying new PTM-related codes.
The team also studied the role of enzymes in inducing or removing PTM in existing signaling systems where the root cause of protein degradation and production was unknown due to the diversification of new PTM-related codes. They suggested that the origin of disease-related protein lifespan changes be digitized and identified in advance with PTM codes to change the focus of ubiquitin signaling.
This is because ubiquitination is rarely the direct point of regulation, but rather preceded by the addition or removal of other PTM signals at or near the ubiquitination site, explained one of the researchers.
Professor Lee Ji-min at the Graduate School of Medical Science & Engineering of KAIST said, "The newly proposed PTM-activation or PTM-inactivated degron code is expected to improve the diagnosis and drug development for various diseases, like cancer or neurodegenerative diseases, that do not respond to existing drugs or develop resistance."
The study was published in the Nature Communications journal on Jan. 13.

