A team of Korean researchers has revealed the function of the Dicer protein in generating microRNA (miRNA) for developing RNA therapeutics in two studies published simultaneously in the Nature journal.
In particular, the first study highlighted how human dicer (hDICER) recognizes the precursor microRNAs (pre-miRNAs) with high specificity to reveal the pathogenesis of hDICER-related diseases like cancer.
The second study demonstrated how the Dicer protein is recognized by a substrate for the potential design of RNA therapeutics.
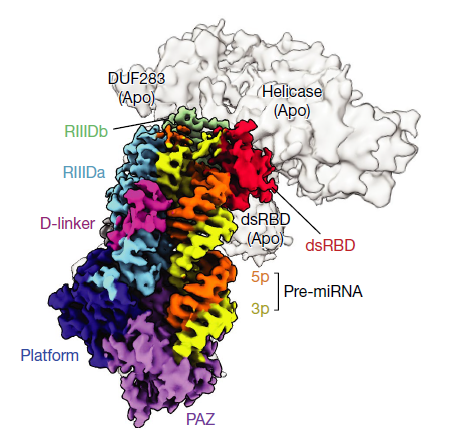
The team, led by Professor Vic Narry Kim of RNA research at the Institute for Basic Science and Professor Roh Soung-hun of Seoul National University’s Molecular and Biology Institute, also identified the three-dimensional structure of Dicer “for the first time in the world” using cryo-electron microscopy to capture the moment when the hDICER cleaves the pre-miRNA.
Briefly summarised, miRNA is a small RNA composed of about 22 nucleotides that bind to messenger RNA that produces proteins and selectively suppresses the expression of specific genes.
By regulating this gene expression, miRNA can directly or indirectly affect cell proliferation and differentiation, immune response, aging, and disease, the researchers said.
Also, these miRNAs exist abundantly in the body and are produced through a unique process in which pre-miRNA is sequentially cleaved by DROSHA and Dicer proteins.
Accordingly, the researchers synthesized over one million pre-miRNAs to confirm Dicer's working principle and identify how pre-miRNAs are cleaved to better understand the functions which this protein controls.
Subsequently, they successfully identified the sequence necessary for Dicer to cleave the precursor via large-scale analysis and named it, the GYM motif.
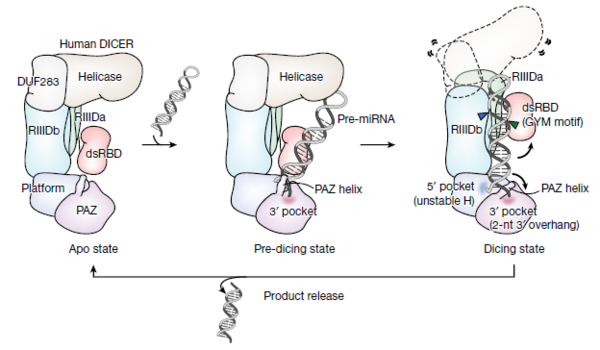
The researchers confirmed that the GYM motif helps Dicer determine the site of pre-miRNA cleavage. Additionally, they demonstrated that the GYM sequence can greatly improve RNA interference (RNAi) efficiency in the areas of different viral infections, rare diseases, neurological disorders, and many types of cancers.
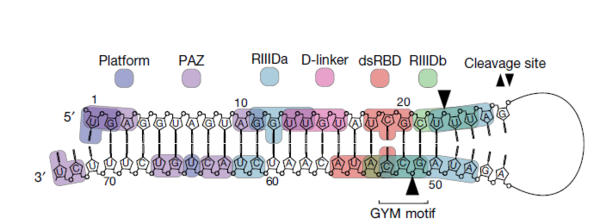
In addition, the researchers discovered that some cancer patients have mutations in a specific part of Dicer, and when this mutation occurs, they cannot properly recognize pre-miRNA, which affects miRNA production.
“Understanding the process of miRNA generation can help identify the causes of disease and improve gene therapies by increasing the efficiency of RNAi,” Director Kim said. “Thanks to the continuous research support we received, we were able to expand our knowledge of the miRNA generation process and are now closer to developing RNA treatments, maybe even in the next 10 years.”
Both studies were published on Wednesday in the latest issue of the Nature journal.

