Prestige Biopharma said on Thursday that its new pancreatic cancer antibody drug candidate, PBP1510, (ingredient: ulenistamab), was granted FDA Fast Track designation.
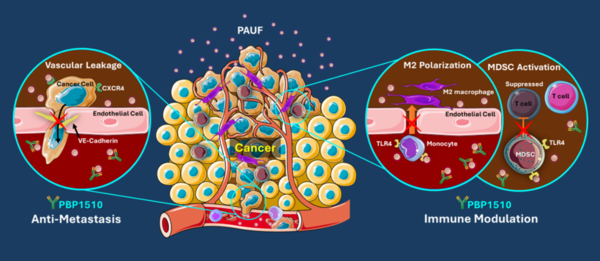
PBP1510 specifically binds to PAUF and exerts anti-metastasis and immune modulation effects. Pancreatic cancer generally expresses a high level of PAUF, a protein that drives cancer cells to spread through blood flow and escape immune recognition.
Consequently, pancreatic cancer has a 5-year survival rate of less than 10 percent and is difficult to detect early because most of the cases are diagnosed late when cancer becomes terminal, so diagnosis and treatment are urgently needed.
Prestige Biopharma's strategy is to speed up the development of a new pancreatic cancer antibody drug PBP1510 and a diagnostic kit that can detect pancreatic cancer early to complete the pancreatic cancer treatment ecosystem.
PBP1510 was also designated as an orphan drug by three regulators in 2020 including the FDA, the EMA, and the MFDS in Korea.
Accordingly, the designation offers the benefits of reduced commercialization time as it can simultaneously prepare for approval applications along with clinical development. In particular, the company plans to actively utilize the rolling review benefit, which allows data from Phase 1/2a clinical trials currently underway in Europe and the U.S. to be reviewed sequentially whenever they become available, said a company official.
Additionally, the designation is expected to accelerate the development of clinical trial design and biomarker utilization as well as consultation meetings related to securing data necessary for item licensing.
"This Fast Track designation is the result of our two-track strategy to conquer pancreatic cancer via early diagnosis and treatment,” said Prestige Biopharma CEO Park So-yeon. “We plan to speed up the development and commercialization of PBP1510 using this designation,”

