On Thursday, two Korean companies specializing in medical artificial intelligence (AI) solutions, Coreline Soft and JLK, revealed their expansion into the South American market.
Coreline Soft announced its entry into Brazil, while JLK announced its entry into Argentina.
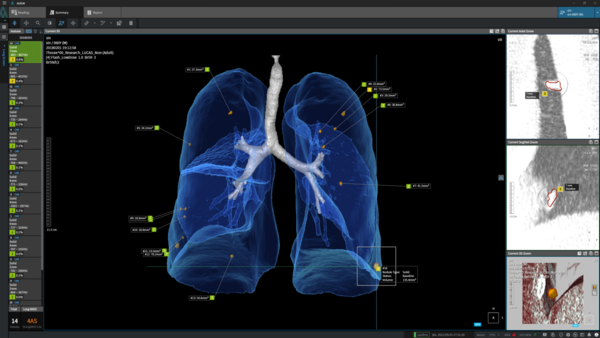
Coreline Soft, which is preparing to be listed on the Kosdaq market this year, closed its pre-IPO with $10.6 billion. The company said it is leading the medical AI market with AVIEW LCS PLUS, the “world's first” simultaneous diagnosis solution for chest diseases licensed at home and abroad.
Coreline Soft also achieved pre-market approval from the Brazilian Health Regulatory Agency (ANVISA) for its AVIEW portfolio which comprises AVIEW LCS, AVIEW COPD, AVIEW CAC, AVIEW Modeler, and AVIEW RT ACS for diagnosing lung-based defects including lung nodules, a chronic obstructive pulmonary disease, and coronary artery calcification.
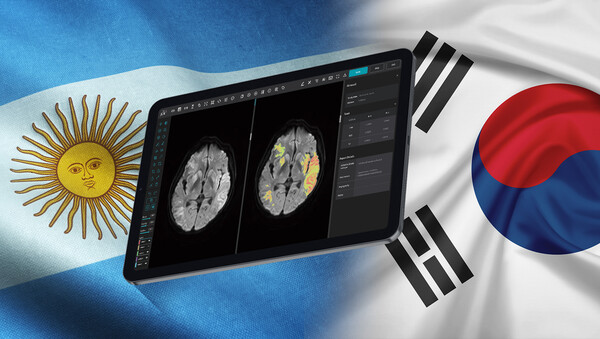
JLK said it holds the “world's largest” number of AI medical device licenses for a single company, with 66 licenses in 11 countries.
In particular, JLK amassed medical device licenses for five of its products from the National Administration of Drugs Food and Medical Devices (ANMAT) in Argentina. JLK's licensed products include JVIEWER-X, a chest radiology image analysis solution based on artificial intelligence, ATROSCAN, a brain aging and dementia progression analysis solution, JBA-01K, a brain aneurysm detection solution, JBD-01K, a breast tumor detection solution, and JBS-04K, a brain hemorrhage detection solution.
ATROSCAN is a solution that provides quantitative and visual information on the volume and cortical thickness by quickly and accurately analyzing brain MRI images. It is actively used for brain age screening at leading health checkup centers in Korea, including Seoul National University Health Center.
The Covid-19 pandemic has increased interest in Korean medical devices, making it easier for Korean companies to enter the Argentine medical market, said a JLK company official. He also added that the ANMAT has also eased regulations for Korean medical devices this year thus reducing the time for product registration in Argentina with a Certificate of Free Sale (CFS).
"The approval in Argentina serves as a bridgehead for us to enter the Latin American medical device market," said JLK CEO Kim Dong-min, "In the case of stroke, the ‘golden hour’ for treatment is crucial and our AI medical solution can help solve this problem."
Both companies are seeking to continue global expansion with their medical AI solutions. The five solutions, including the chest X-ray solution JVIEWER-X and the breast image analysis solution JBD-01K, have already been approved in Thailand and Australia, including the European CE. Coreline Soft has also received clearance from health authorities in the United States, Europe, Japan, Taiwan, Singapore, and Australia.
"Acquiring this license is the cornerstone for expanding the Latin American market, and we will proceed in earnest with entry and sales strategies in Brazil, the largest market in Latin America, and other South American countries," said Coreline Soft CEO Kim Jin-kook.
Related articles
- Coreline Soft’s annual sales nearly doubled in 2022
- Coreline Soft’s brain hemorrhage-detecting software wins approval in Korea
- Korean AI medical imaging companies actively entering US market
- Coreline Soft to supply COPD diagnostic software to US firm
- Coreline Soft passes preliminary screening for listing on Kosdaq
- Coreline Soft aims to debut on Kosdaq through merger in September
- 'Coreline Soft's pseudonymization demand up after government's data security announcement'
- JLK partners with Harvard Medical School to study stroke AI solutions
- Will JLK shed stigma of stock sell-off with US market entry?

