Coreline Soft said on Wednesday that its brain hemorrhage image detection and diagnosis assistance software, AVIEW NeuroCAD, obtained approval from the Ministry of Food and Drug Safety (MFDS) under the government’s innovative medical device integrated review and evaluation system.
The integrated examination and evaluation system for innovative medical devices allows medical devices to be approved so they can quickly enter the medical field reducing the turnaround time from 390 days to 80 days.
Under the integrated review system, devices are evaluated by the Health Insurance Review and Assessment Service (HIRA) for insurance coverage eligibility and by the National Evidence-based Healthcare Collaborating Agency for innovative medical technologies. Then, the MFDS grants medical device approval.
Coreline Soft’s AVIEW NeuroCAD was designated as an innovative medical device in 2021.
With the latest approval, the product will be available in the medical field as a non-insured item.
According to data from the National Statistical Office, the nation's death toll from cerebrovascular diseases, including strokes, stood at 42.6 per 100,000 people in 2021, the fourth largest after cancer, cardiovascular disease, and pneumonia.
Stroke can be largely divided into cerebral infarction caused by blockage of cerebral blood vessels and cerebral hemorrhage caused by rupture of cerebral blood vessels. Thus, diagnosis and emergency measures are more important for cerebral hemorrhage.
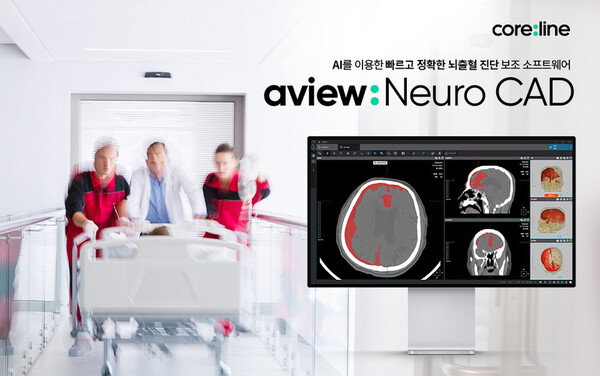
In this regard, AVIEW NeuroCAD supports reading images within a limited time. It presents reading priorities for emergency patients by classifying patient groups with high bleeding, providing priority lesion information according to the amount of bleeding, and a preview of suspected brain hemorrhaging. Additionally, it is linked with the picture archiving system (PACS) of medical institutions along with 2D and 3D image comparison.
"This approval is the fruit of continuous technological advancement and a deep understanding of the clinical field," Coreline Soft CEO Kim Jin-kook said, "We will try to continue expanding our leading influence and new achievements in the field of medical AI."
The company also said it recently requested a preliminary review for listing and plans to go public within this year.
Related articles
- Coreline Soft’s AI-backed lung nodule detector gets FDA 510(k) clearance
- Coreline Soft wins Singapore regulator approval for 9 of its AI solutions
- Coreline's AI-based oncology contouring device wins FDA 510(k) clearance
- IBS maps brain circuits using non-invasive beam projector
- Coreline Soft’s annual sales nearly doubled in 2022
- 'Efficient stroke prevention requires dedicated stroke control center'
- Coreline Soft, JLK flood South American market with medical AI solutions
- Coreline Soft to supply COPD diagnostic software to US firm
- Coreline Soft aims to debut on Kosdaq through merger in September

