As expensive new anticancer drugs keep coming, the government must revise the current financial sharing system that offers excessive patient benefits to improve health insurance’s finance and expand patients’ access to treatments.
Professor Kim Seong-bae of the Oncology Department at Asan Medical Center said so during the Global Breast Cancer Conference (GBCC) 2023 at Great Walker Hill Seoul last Saturday.
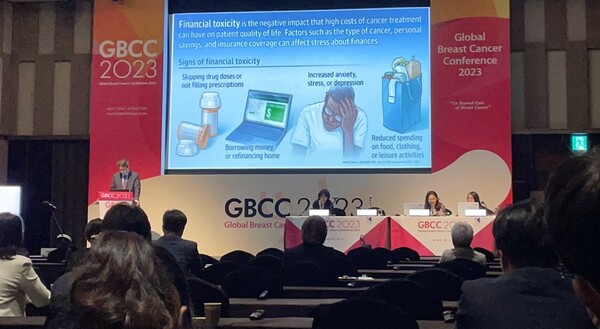
In a session titled, “The Cost of newly approved drugs: Can we afford them?,” Profess Kim spoke about access to breast cancer treatments in Korea. During his presentation, Kim stressed the “value of new drugs,” which can lower death rates with improved treatment effects, return patients to everyday life more quickly, and eventually reduce social costs.
“The use of new drugs reduces hospitalization, brings patients back to daily life, lowers medical costs, and offers social and economic effects,” he said.
Kim noted that in the Netherlands as an example where 18-30 percent of head and neck cancer patients went back to everyday life within six months, adding that in France and Japan, too, 82 percent and 81 percent of breast cancer patients returned to daily life within a year.
In contrast, access to new drugs ranked 19th in 31 advanced countries (regarding drugs released in 2005 and after). Notably, the reimbursement rate fell as time went by, and it took a considerable time to receive benefits, leaving much room for improvement, Kim pointed out.
“In Korea, new drugs must undergo a series of phases to reach patients, such as ‘approval,’ ‘market release,’ and ‘reimbursement.’ Among them, granting insurance benefits or not is the most significant factor determining access to new drugs,” Professor Kim said. “Besides, the ‘financial toxicity’ latent in drugs also heavily influences access to new drugs. High drug costs lower patients’ treatment compliance, and debt caused by treatment triggers stress and anxiety and not eating properly, adversely affecting their quality of life.”
Thus, Kim cited “reimbursement” and “financial toxicity” as critical elements in access to new drugs in Korea.
“Increase in medical expenses due to populating aging will bring about considerable costs on the Korean health insurance’s finance,” Kim predicted. “When expensive new drugs increase and reimbursement environment aggravates, Korea must supplement its insurance system to increase access to new treatments.”
Kim pointed out that in Korea’s health insurance system, patients have only to pay 5 percent of medical costs once diagnosed to have cancer.
“When expensive new drugs increase, the government must raise patients’ burden to help reduce the health insurance’s financial burdens,” stressing the need to revise the “special calculation system for cancer patients.”
He also mentioned other factors to increase access to new drugs, such as replenishing and educating personnel responsible for approving new drugs and deliberating reimbursement for them at the Ministry of Food and Drug Safety and the Health Insurance Review and Assessment Service. Kim added that the government must also raise the examination fee, which stands at a mere 2 percent of other advanced countries, to a more realistic level.
In addition, Kim presented ways to make evaluation standards for new drugs’ cost effectiveness more flexible and raise separate financial sources for expensive drugs.
To do so, he proposed patient organizations and medical experts participate in deliberation for reimbursement and put the issue into public discussions at various interdisciplinary conferences.
In the ensuing debate session, Professor Ahn Hee-kyeong of the Department of Oncology at Gachon University Gill Medical Center introduced examples of new drugs that successfully entered the reimbursable sphere aided by patients’ opinions.
“We already have two cases of positive experience where we could use new drugs quickly with patient support,” Ahn said. “When Perjeta (pertuzumab) was introduced 10 years ago and failed to receive reimbursement for three years, an online patient organization petitioned the government and won reimbursement for the primary treatment.”
Professor Ahn also cited the case of Enhertu (T-DXd), a prohibitive breast cancer drug that doctors badly wanted to use because of its remarkable progression-free survival (PFS).
“Patients also knew about this drug and made a quick national petition based on their experience of pertuzumab,” Ahn said. “The drug is now under review, and we all hope it will receive reimbursement without a hitch.”
Related articles
- Enhertu cements its position as standard 2nd treatment for HER2+ breast cancer
- Daiichi Sankyo's Enhertu passes cancer drug benefit review after patients’ petition
- Breast cancer patients increased by 30.5% in 5 years: report
- Enhertu recognized in Asia, too, as essential targeted agent for breast cancer
- ‘Socioeconomic benefit of Enhertu estimated to be ₩260 bil.’

