“Enhertu (trastuzumab deruxtecan), an antibody-drug conjugate, demonstrated strong cell toxicity effects, bringing about new changes in HER2-positive metastatic breast cancer treatment that had had unmet clinical needs. Based on efficacy and safety in various clinical trials, Enhertu has already taken a firm position in the standard secondary treatment in the clinical field.”
Professor Kim Min-hwan of the Oncology Department at Severance Hospital said so during the Global Breast Cancer Conference (GBCC) 2023 at Grand Walker Hill Seoul last Saturday.
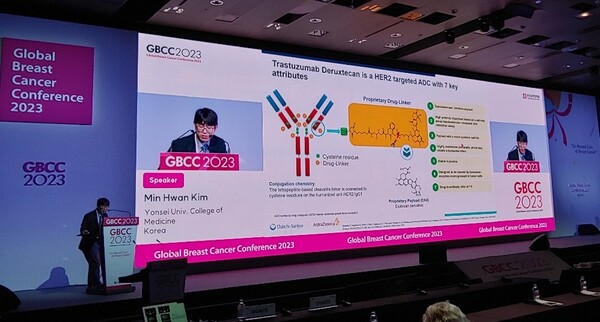
In a presentation titled “Enhertu, the game changer in HER2+mBC treatment: from clinical trial to clinical practice,” Professor Kim highlighted Enhertu’s mechanism, its efficacy and safety demonstrated in clinical trials, and its benefits for domestic clinical treatment.
“With the recent development of cancer biology, various targeted agents are also being developed to treat cancer,” Kim said. “Notably, antibody-drug conjugates (ADCs) are getting over a set of limitations that could not be overcome by existing monoclinic antibodies or low molecular targeted agents and are showing promising results in various cancers through new treatment mechanisms targeting cancer cell membrane proteins.”
Kim noted that HER2+ breast cancer is refractory due to frequent drug resistance, although there had been various HER2 targeted agents, such as trastuzumab, pertuzumab, and lapatinib, leading to patients’ death even after receiving several systemic anticancer chemotherapies.
“However, the emergence of ADCs has brought about new changes in the treatment of HER2+ metastatic breast cancer that had high unmet clinical,” he said. “Notably, Enhertu showed high cell toxic effects owing to high drug-antibody ratio through a linker-payload system.”
Professor Kim then shed light on Enhertu’s efficacy and safety proven through Destiny Breast01, Destiny Breast02, and Destiny Breast03 studies, phase 2 and 3 clinical trials on HER2+ metastatic breast cancer patients.
In the Destiny Brast01 study, the clinical trial that provided the basis for Enhertu’s winning conditional approval in Korea, Enhertu’s objective response rate (ORR) in HER2+ metastatic breast cancer patients with previous treatment experiences was 60.9 percent, and its progression-free survival was 16.4 months.
“Enhertu’s innovative treatment effects were reaffirmed in Destiny Breast02 and Destiny Breast03 studies,” Kim said. “Based on PFs, ORR and complete response rate (CRR) and effects on patients with a metastatic brain tumor, Enhertu took a firm position as the standard secondary treatment for HER2+metastatic breast cancer patients.”
In the Destiny Breast02 study, researchers compared and evaluated Enhertu and control groups selected by clinicians (trastuzumab/capecitabine or lapatinib/capecitabine) in HER2+ breast cancer patients with treatment experiences using trastuzumab emtansine (product name: Kadcyla). Enhertu showed a superior ORR of 69.7 percent compared to the control group’s 29.2 percent, PFS of 17.8 months vs. 6.9 months, and overall survival (OS) of 39.2 months vs. 26.5 months.
In the Destiny Breast03 study, researchers compared and evaluated Enhertu and trastuzumab/emtansine in HER2+ progressive breast cancer patients with treatment experiences using trastuzumab- and taxane-based chemotherapies, which drew attention because Asians accounted for 60 percent of subjects and those with metastatic brain tumor accounted for 20 percent.
"In this study, Enhertu showed a median PFS value of 28.8 months, about four times longer than the trastuzumab/emtansine. This is far longer than the PFS of 18.7 months confirmed at the CLEOPATRA study, the clinical trial for primary treatment,” Professor Kim emphasized.
The result of additionally analyzing the Destiny Breast03 study released at the San Antonio Breast Cancer (SABC) 2022 last December, the one-year survival rate was 94.1 percent in the Enhertu group and 86 percent in the trastuzumab/emtansine group. Their two-year survival rates were 77.4 percent and 69.9 percent.
“Notably, Enhertu showed an intracranial response rate of 63.9 percent, far higher than trastuzumab/emtansine, confirming excellent effects in patients with a metastatic brain tumor,” Kim said, introducing it proved similar effects in the clinical field by citing the examples of some patients with a metastatic brain tumor.
“Regarding safety, 51 patients, or 20 percent, stopped taking the drug as they showed abnormal responses, including interstitial pneumonia (6 percent), interstitial lung disease (5 percent), and pneumonia (2 percent),” he said. “It is essential to manage patients carefully to detect pneumonia in the early phase to maintain treatment with Enhertu.”
Kim noted that based on these data, Enhertu won approval from the Ministry of Food and Drug Safety to treat unresectable or metastatic HER2+ breast cancer patients who received one or more HER2-based chemotherapies last December, starting an important mission to save patients’ lives.
“If you search Enhertu these days, many comments are wanting the government to provide insurance benefits for Enhertu,” Professor Kim said. “The academic community also expects there will be progress in the government’s policy in Enhertu’s treatment environment, and hope many patients will benefit from it.”
Meanwhile, the cancer disease deliberation committee under the Health Insurance Review and Assessment Service (HIRA) will re-discuss Enhertu’s application for reimbursement scheduled for Wednesday. In addition, Daiichi Sankyo Korea, responsible for Enhertu’s domestic sale, has reportedly submitted an additional financial sharing plan to HIRA, drawing attention to the deliberation’s result.
Related articles
- ‘Korea must raise cancer patients’ burdens to expand their treatment access’
- Daiichi Sankyo went all out to secure reimbursement for Enhertu. The result?
- Tagrisso gets wider insurance benefits, but Enhertu needs more discussion
- Cancer patients ask lawmakers for insurance coverage of expensive Tagrisso, Enhertu
- Daiichi Sankyo's Enhertu passes cancer drug benefit review after patients’ petition
- NCC’s novel anti-cancer fatty acid oxidation inhibitor OKed to begin clinical trials
- Breast cancer patients increased by 30.5% in 5 years: report
- Enhertu recognized in Asia, too, as essential targeted agent for breast cancer
- ‘Socioeconomic benefit of Enhertu estimated to be ₩260 bil.’

