BOSTON, Mass. -- By Lee Han-soo/Korea Biomedical Review correspondent -- In today's fast-paced and highly competitive pharmaceutical industry, companies are constantly seeking ways to accelerate innovation and bring new therapies to market.
Licensing agreements have become a vital strategy for fostering collaboration and maximizing the potential of intellectual property.
Merck, a renowned global pharmaceutical company, has established a robust licensing division, playing a pivotal role in driving innovation and expanding its reach in the healthcare sector.
In particular, the immunology field has become increasingly competitive, with original powerhouses -- Janssen, Sanofi, and AbbVie, and more recently, Merck, Pfizer, Lilly, and BMS -- aggressively expanding their portfolio through acquisitions and license agreements.

Against this backdrop, Korea Biomedical Review met with Sehyun Jason Kim, Director of Immunology Search & Evaluation at Merck Business Development (BD) & Licensing, to talk about Merck's licensing process.
Kim evaluates hundreds of drug candidates in the field of immunology for Merck annually.
"Considering Humira was the world's bestselling drug before its loss of exclusivity (LOE), immunology has a large market and has the potential to expand," Kim said. "In the U.S. alone, about 7-8 percent of the population still suffers from autoimmune diseases, and with the life expectancy rising, more people are suffering from autoimmune and inflammatory diseases."
However, while there is such unmet medical need, immunology is a field that has yet to see much innovation compared to other fields, he added.
"For example, combination therapy and companion diagnostics, which is now considered a standard practice in the field of oncology, has just started to be applied in the immunology field," he said. "As a result, a lot of innovation is happening in the immunology field, making it an attractive market."
When asked how Merck decides on what drugs to search and evaluate, Kim stressed that Merck puts the utmost focus on science.
"Merck puts a lot of focus on science when considering licensing-in candidates," Kim said. "What this means is that the science backing up a candidate has to make sense from start to finish and expand to clinical data."
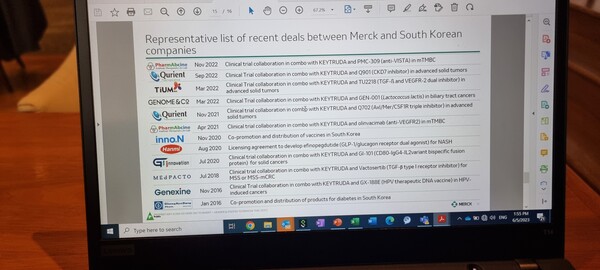
The company conducts due diligence on the candidate only after it confirms the science behind the drug substance, he added.
According to Kim, the hardest part of his job is to conduct internal buy-in, which is getting stakeholders, such as internal team members, to persuade the leadership team to approve the license or acquisition agreement, and science is the most crucial factor.
"After the approval from our leadership team, we aggressively conduct the licensing deal or acquisition," he said. "The recent acquisition of Prometheus Biosciences was a good example of such a process."
Kim stressed that the company was interested in acquiring Prometheus Biosciences, a Nasdaq-listed biotech firm, a few years ago.
After Prometheus reported promising results of its candidate during the 2023 J.P. Morgan Healthcare Conference in January, the company's plans to acquire Prometheus accelerated, he said.
"We received the buy-in approval from our leadership team in March to April, and we completed the due diligence process and the acquisition agreement in a mere two weeks."
Kim also stressed that Merck's search and evaluation team differs from its competitors as the entire team has a background in research.
"For example, Christopher Mortko, who is the head of our search and evaluation unit, worked as a researcher after receiving his Ph.D. from UCLA, Ronald Kim, who is in charge of search and evaluation for candidates treating cardiometabolic diseases, renal, respiratory, and ophthalmology joined the team after working as a research for 25 years, and I also worked as a researcher in Genentech," Kim said.
Pros and cons in Korean drug development
Before joining Merck, Kim also worked with ABL Bio, where he was a key member in licensing out ABL301, a treatment for Parkinson's disease, to Sanofi last year.
Kim stressed that his experience in Korea showed that Korean companies are very nimble in making decisions.
"In the case of Korean companies, it seems that as soon as they realize that the other party has a need, actionable items are created and can be executed immediately," he said. "We were able to do the deal with Sanofi thanks to such nimbleness."
Kim said that the deal with Sanofi did not just happen as soon as ABL Bio met them. It took over a year.
"But from the first time we met, we kept interacting with their team and leadership," he said. "We also got a lot of feedback, and we kept refining our assets based, and I think this is what led to Sanofi signing the license deal with ABL Bio."
When asked about the cons of Korean drug development companies, Kim said that Korean drug development companies in the past seemed to be more focused on making money rather focusing on the actual drug development.
However, a lot has changed since, and he has recently seen Korean companies more focused on developing good drugs based on science and addressing unmet medical needs, he noted.
Kim also spoke about his thought on Merck.
"The company is a very traditional company, and our goal is clear, we aim to make good drugs that can benefit patients the most," he said. "While the company prides itself on developing good drugs through internal research, we also put a lot of focus on open innovation to expand our pipeline."
Therefore, the company's motto of "Curiosity inspires us to invent for life" is very befitting, he added.
Domain expertise most important for a career in BD
During the interview, Kim also gave some advice to Koreans who seek a career path in BD.
"The most important for people seeking a career in BD for a global pharmaceutical company is to have domain expertise, which means requiring a science background," he said. "In the case of Merck, the company views science expertise as one of the most crucial factors in employment, so I recommend a degree in the field."
Making publications in scientific journals can also be very helpful, he added.
Kim also stressed that spending time as an analyst at securities firms could also help boost a person's BD career.
"It's really important to understand the market and to be able to take that and analyze companies in the field and figure out where the market is going," he said.
However, Kim also warned that people who only see the glamor side of BD should rethink their career path.
"A lot of Koreans that want to work in BD have told me that they want to work in the field as the BD unit serves as the face of the company, which seems glamorous," he said. "While it is true the job is very rewarding, it comes at a cost as BD employees have to put a lot of their time and effort, which means that you don't have a lot of time to spare."
Kim also stressed starting a BD career in a Korean company may be more beneficial.
"While I started my career at a multinational pharmaceutical company, that does not mean that my time at ABL Bio was not beneficial as it allowed me to grow my networking skills and stature," he said. "For example, I had the chance to meet with a lot of Merck's R&D leadership team during my time at ABL Bio, and this helped me a lot when applying for the BD role at Merck."
According to Kim, another benefit of starting a career at a Korean company is the amount of experience a person can gain in licensing out a drug.
"As I have mentioned, 'buy-in' of the leadership team is the most important part when licensing in a drug for a pharmaceutical company, and such a process is somewhat similar to licensing out a drug," he said. "As I had honed my experience in licensing out drugs at ABL Bio, I regularly apply the experience that I have gained when licensing out a drug during the buy-in process."
Related articles
- Samsung Biologics’ Plant 5 will be ready 5 months earlier than scheduled: CEO
- [Photo News] 2023 BIO International Convention rekindling success as attendees flock back after Covid-19 pandemic
- [Special] Shaperon’s exclusive technology to fuel global expansion
- [Special] EuBiologics to spearhead ‘K-vaccine’ wave at BIO 2023
- Lotte Biologics to highlight commitment to high-quality services at BIO 2023
- [Special] Dong-A ST speeds up global entry with new drugs
- Samsung Biologics to participate in 2023 BIO International Convention
- Prestige Biopharma to attend BIO 2023
- CHA Biomedical Group seeks global biz expansion at BIO 2023
- [Special] Korean CDMO companies ready to wheel and deal at BIO 2023
- [Special] Which Korean companies will be most active at BIO 2023?
- Samyang Holdings Biopharm to open biodegradable suture plant in Hungary

