In a shocking revelation, it has come to light that Minoxidil Tab. Hyundai, the company's hypertension treatment which is more famous for its off-label use as a hair loss treatment, has inadvertently been labeled as Tamirin ER Tab., a dementia treatment, and given to dementia patients.
The impact of mislabeling goes beyond mere inconvenience, as patients suffering from dementia already face numerous challenges, and the last thing they need is the added risk of taking the wrong medication.
Hyundai Minoxidil, originally designed as a treatment for severe hypertension, gained fame for its alleged hair restoration capabilities. It was often prescribed off-label for this purpose, despite lacking official approval as a hair loss remedy.
The Ministry of Food and Drug Safety (MFDS) first received notice of the mix-up after receiving a report from a vigilant pharmacist.
Hyundai Pharmaceutical took immediate action to recall the affected batches of Minoxidil Tab. Hyundai.
According to the MFDS, the recall encompasses 19,991 bottles of the product, specifically those with product number 23018. These bottles, containing 30 tablets each, were manufactured on May 15, 2023, and their expiration date is May 14, 2026.
The MFDS also clarified that the two drugs were not mixed together but were packaged incorrectly and that it appears that both drugs were being produced on the same assembly line, leading to this bizarre mix-up.
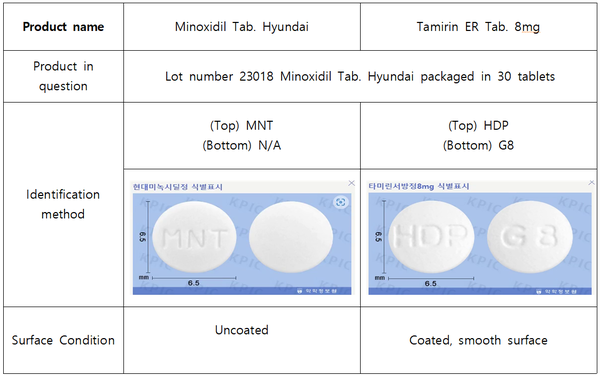
The MFDS provided instructions on how to identify the two drugs.
"The Minoxidil Tab. Hyundai can be recognized by the engraving 'MNT' on the top surface, while the Tamirin ER Tab. 8mg are imprinted with HDP," the ministry said. "Additionally, Minoxidil Tab. are uncoated pills, whereas the Tamirin ER Tab. has a smooth, coated surface."
A spokesperson for the MFDS warned individuals who were prescribed or taking these medications to exercise extreme caution and verify the drugs before use.
While only one incident has been reported thus far, involving a single container of 30 tablets, the MFDS has expressed grave concern over this packaging error.
The regulatory body is currently conducting a thorough investigation into the company responsible for this negligence and has vowed to make every effort to prevent the recurrence of such incidents and ensure the distribution of safe pharmaceutical products in the future.
As for potential administrative sanctions, the MFDS stressed that it is currently difficult to predict the extent of any penalties.
"While the company claims that only one container was affected, a comprehensive assessment of the nature of the error and any associated violations must be conducted before reaching a final decision," the ministry’s spokesperson said. "Trusting the company's statements alone is insufficient at this stage."
However, the spokesperson added that it is important to note that pharmaceutical products are subject to good manufacturing practice (GMP) regulations, and any violations of these standards will certainly be addressed.
"The severity of the penalty will depend on the specific nature of the negligence," he said. "Therefore, it is crucial to closely monitor the situation and await further developments."
On the drug mislabeling news, shares of Hyundai Pharmaceutical reached a 52-week low.
As of 10:30 a.m. on Monday, the company's shares stood at 3,925 won ($2.99), down 4.73 percent from the previous day.

