A mislabeling incident occurred in which Celltrion Pharm’s tablets were packaged in Myungmoon Pharm’s medicine containers. Theragen Etex, the contract manufacturer of the product in question, explained that it was a simple mix-up due to an employee’s mistakes.
According to the Ministry of Food and Drug Safety (MFDS), Myungmoon Pharm is recalling some batches of its over-the-counter drug "Myungmoon Aspirin Enteric Coated Tablets 100mg (aspirin)" (manufacturing number 22004). That’s because the containers of this product are filled with Celltrion Pharm’s Astection Enteric-Coated Tablets 100 mg (aspirin). Both products have the same active ingredient and content.
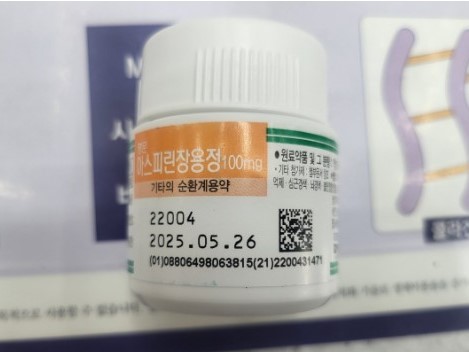
The products' manufacturing date targeted for recall is May 27, 2022, and the recall size is not yet known. The packaging units are 30- tablets and 500-tablet bottles. Myungmoon has publicly announced the recall on its website. The drugs will be collected by visiting dealers and medical institutions or taking them back from pharmacies and hospitals.
"Distributors, pharmacies, medical institutions, and others who have the medicines in storage are requested to immediately stop selling them and return them to the person responsible for the recall," the company said.
Both products are outsourced to Theragen Etex. They are manufactured at the company’s Ansan Plant in Gyeonggi Province. The company claims that the incident was a simple case of medication mixing due to employee error and that the scale of the mispackaging was not large.
"It is a simple mixed packaging. We understand that a new employee made a mistake. Myungmoon takes no additional measures against us,” a Theragen Etex official said over the phone.
The number of recalls by domestic pharmaceutical companies due to adulteration or mispackaging of medicines has increased recently. Experts point out that frequent adulteration incidents are threatening the health of patients and consumers.
In June, for example, Hyundai Pharm created a stir by distributing the dementia drug Tamirin ER Tab 8mg in the container of its hypertension drug Minoxidil Tablet (minoxidil). Earlier this month, KORUS Korea conducted a seller’s recall of its antibiotic KORUS Cefaclor Cap 250mg (export name KEFACCAP) by attaching the label of a different packaging unit.
After the incident with Hyundai Pharm's Minoxidil tablets, the Citizens United for Consumer Sovereignty issued a position paper, pointing out that it was a "serious problem caused by the apparent failure to observe quality control standards," urging the pharmaceutical industry to reflect and take measures to prevent recurrence.
Related articles
- ‘Continuous drug mixing incidents threaten consumer health’
- Hyundai Pharm accidentally labels hypertension drug as dementia treatment, endangering patients
- Celltrion, Celltrion Healthcare to repurchase shares worth $145 bil. to boost shareholder value
- Celltrion to spend $95.3 mil to build new drug product factory in Songdo

