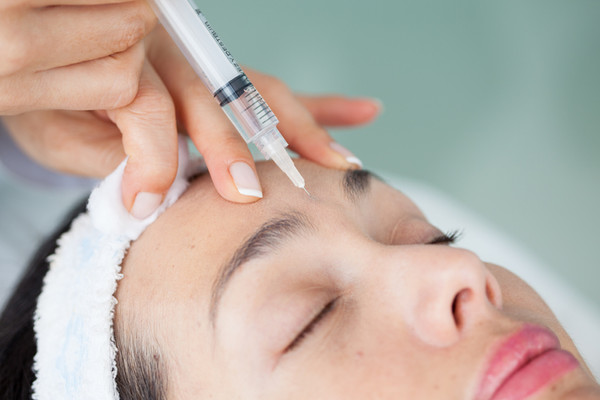
Huons Biopharma, a company specializing in botulinum toxin (BTX), announced Thursday that it has received approval from the Ministry of Food and Safety (MFDS) for the phase 3 Investigational New Drug (IND) clinical trial of its HU-045, a BTX, treating frown lines.

HU-045 was developed to address moderate or severe frown lines. The phase 3 clinical trial, which will assess safety and efficacy, is set to take place at three hospitals in Korea, including Samsung Medical Center, and will include adults aged 19 and older.
In January 2020, Huons Biopharma conducted a phase 1 clinical trial of HU-045, followed by a phase 2 trial in December 2021, which confirmed the efficacy in the treatment of moderate to severe frown lines.
To reduce the potential for resistance, Huons Biopharma eliminated non-toxin proteins and purified the neurotoxin protein to minimize the risk of neutralizing antibody formation.
In addition to obtaining a license for HU-045, Huons Biopharma plans to expand sales of its existing BTX, Liztox (export name: Hutox).
The company has plans for Liztox to further enhance its competitiveness by obtaining approvals for additional indications besides frown lines and crow's feet.
In March, the MFDS approved a phase 3 clinical trial for Liztox for the treatment of benign masseteric hypertrophy (square jaw).
Huons Biopharma operates in 11 countries, including Russia, Iraq, and Kazakhstan. The company is also in discussions with leading companies in the U.S., Europe, and China, where there is a high demand for BTX.
"Following the existing Liztox, we are accelerating the development of next-generation BTX such as HU-045," a company official said, "We plan to complete the phase 3 clinical trial of HU-045 for frown lines improvement within a short period and obtain a license to respond to the growing demand for BTX."

