Huons, a Korean healthcare company listed on Kosdaq, said Monday it has received IND approval from the Ministry of Food and Drug Safety for HUC1-394, a treatment for dry eye disease, to conduct a phase 1 clinical trial.
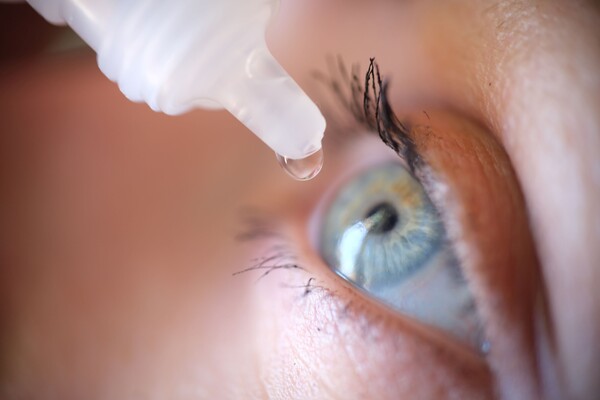
HUC1-394 is a peptide-based eye drop for dry eyes. In 2020, Huons licensed-in the technology for HUC1-394 from Novacell Technology, a peptide R&D company based in Pohang, North Gyeongsang Province. In June 2023, Huons submitted an IND application for HUC1-394 to the Ministry of Food and Drug Safety for a phase 1 clinical trial.
Huons plans to evaluate the safety, tolerability, and pharmacokinetic properties of single and repeated dosing of HUC1-394 in 60 adults.
Peptides are compounds composed of 50 or fewer linked amino acids. They are characterized by their smaller size than proteins. Depending on the type, they play different roles in the body, such as preventing inflammation, activating cells, and stimulating cell production.
Dry eye disease is a condition in which the tear film fails to provide adequate lubrication to the ocular surface, causing it to dry out, resulting in eye discomfort and irritation. Dysfunction in the meibomian glands and the immune system may contribute to a vicious cycle of tear film instability. Key symptoms include itchy, red eyes and increased sensitivity to light.
"This clinical trial will determine the safety, tolerability, and pharmacokinetic properties of HUC1-394 eye drops after administration, and we look forward to providing patients with dry eye disease with a new, novel treatment option," a Huons official said.
Related articles
- Huons Meditech to import Canadian device for prostate cancer treatment
- Huons Meditech awarded $7 mil Export Tower by KITA
- Huons Biopharma gets nod for P3 trial of BTX to treat frown lines
- Huons Global’s Q3 operating profit jumps 75% on strong international sales
- Huons donates 6,000 continuous glucose monitors to type 1 diabetes patients
- Huons licenses in health functional material from EFIL BioScience
- Huons’s 2023 sales expand 12% riding on anesthesia exports to North America
- Crystal Life Sciences changes name to Huons Life Sciences

