Waycen, a medical AI company, said Monday that it has been certified as the “No. 7 Innovative Medical Device Software Manufacturer” by the Ministry of Food and Drug Safety (MFDS).
Waycen won the certification for its continuous quality management efforts, including product development, verification, and maintenance, and its performance in risk management processes, including software problem resolution.
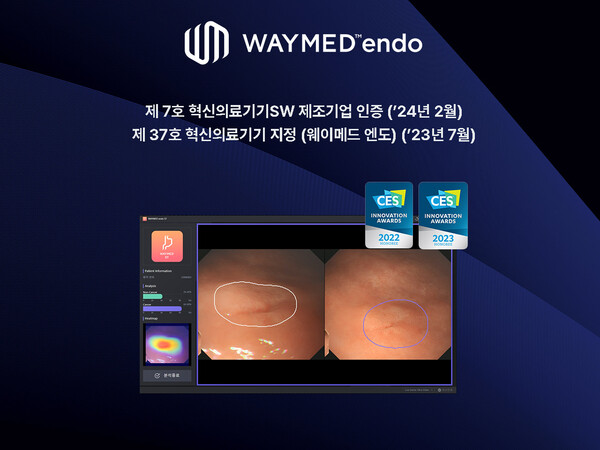
Last July, the company’s Waymed Endo, an AI gastroscopy and colonoscopy medical device software, was also designated as the 37th innovative medical device by the MFDS.
Waymed Endo is the first medical software in Korea that analyzes gastroscopy and colonoscopy in time through artificial intelligence.
Beyond the lesion detection function, it can provide suspected areas of gastric cancer and the probability of gastric cancer to medical staff to assist in diagnostic decisions. It has recently been supplied to the National Health Insurance Service Ilsan Hospital, Veterans Health Service Medical Center, and Gangneung Medical Center.
The Innovative Medical Device Software Manufacturer Certification System evaluates the safety management level of medical device software manufacturers to certify excellent manufacturers and supports rapid commercialization by exempting some data when applying for permits. Certified companies are exempted from submitting data, including comparison data with already approved products, data on the purpose of use, and the principle of action when applying for a medical device item license.
Earlier, the MFDS also selected medical AI companies with excellent product development skills, including Lunit, VUNO, and Corelinesoft, and certified them as innovative medical device software manufacturers.

