Coreline Soft's AVIEW Aorta has been recognized as an innovative medical device with insurance benefits, broadening its application in emergency medicine and establishing itself as a new source of revenue.
On Monday, the medical AI company announced that AVIEW Aorta, a class 3 cardiovascular image detection and diagnostic auxiliary software, had been officially designated as an innovative medical device under Ministry of Food and Drug Safety (MFDS) Announcement No. 2024-95, dated Feb. 21.
As a result, AVIEW Aorta can be supplied to medical institutions on a non-reimbursed basis or with selective reimbursement for at least three years to establish clinical evidence of effectiveness. After another evaluation of the new medical technology, the product will be officially registered and put to market with (temporary) reimbursement.
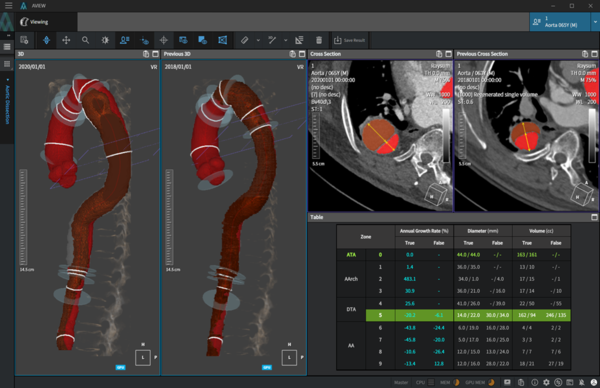
AVIEW Aorta is designed to automatically detect aortic dissection using AI, aiding in diagnosis. The aorta, being the body's largest and thickest blood vessel, branches off arteries supplying blood to vital organs like the heart, brain, limbs, and internal organs. Aortic dissection occurs when the aorta's lining tears, diverting blood flow to the inner layers, a condition known as an aortic dissection.
If left untreated, aortic dissection is a serious condition with a mortality rate of 1-2 percent per hour in the first 24 hours after symptom onset, making rapid diagnosis and treatment essential. In this situation, AVIEW Aorta can quickly diagnose and triage aortic dissections to help hospitals treat patients within a critical timeframe. In particular, it is possible to send a notification message to the hospital by interlocking with the hospital system, enabling preemptive response and quick communication of medical staff.
In addition, it has been recognized for its high technology and stability as an AI cardiovascular diagnostic solution by obtaining a medical device manufacturing license of class 3. The product's main functions include notification of the presence of dissection and analysis results, divided intra-aortic area notation, measurement of diameter (mm) and volume (cc) according to the area, and the provision of analysis results and access link information. Besides, it can compare 2D and 3D images and interoperate with the medical institution's PACS system.
Coreline Soft entered the non-reimbursement and/or selective reimbursement market in January with AVIEW NeuroCAD, an AI-based solution for diagnosing cerebral hemorrhages. AVIEW NeuroCAD is a solution that automatically analyzes the amount of bleeding in a patient's brain CT image, enabling doctors to read the image and make diagnostic and treatment decisions within a limited time.
After entering the emergency medicine sector with AVIEW NeuroCAD, Coreline Soft plans to build a pipeline of emergency medicine products, including AVIEW Aorta, to provide a medical imaging platform optimized for the end-to-end emergency room experience.
"The innovative medical device designation recognizes our ability to complement existing cardiovascular diagnostic methods and play a significant role in early diagnosis," CEO Kim Jin-kook said. "We are commercializing it as an optimized solution for the emergency environment, and we expect our overall revenue to increase rapidly as additional solutions enter the non-payment market and the number of patients is confirmed."
Related articles
- Coreline Soft partners with UAE healthcare firm MHC to target hospitals in Middle East
- Coreline Soft shares plummet on 1st day of listing
- Coreline Soft expects to break even in 2025
- Coreline Soft partners with HealthCare Konnect to accelerate Swiss expansion
- Korean medical device makers shine at Europe's largest radiology conference
- Coreline Soft wins 2 iF Design Awards for innovation in medical AI solutions
- Coreline Soft's medical AI advances via European hospital partnerships, supply contracts
- Coreline Soft win FDA 510(k) clearance for AI-powered coronary artery calcification analysis solution
- Coreline Soft begins non-reimbursed billing for AVIEW NeuroCAD brain CT analysis solution

