
CHICAGO, Ill. – A breast cancer study outcome was so impressive that it received a standing ovation at the American Society of Clinical Oncology (ASCO 2022), the world’s largest meeting in oncology being held in Chicago.
The standing ovation went to researchers of phase 3 DESTINY-Breast04 trial of Enhertu, a new breast cancer drug.
According to the study, Enhertu (trastuzumab deruxtecan) reduced the risk of disease progression or death by a whopping 50 percent, compared with conventional chemotherapy, in HER2-low metastatic breast cancer.
Experts said that the excellent result opened a new era of breast cancer treatment.
At ASCO 2022, Korea Biomedical Review met with four Korean oncologists to learn about the clinical implication of the DESTINY-Breast 04 study results and the urgent need to introduce Enhertu in Korea. The four oncologists are Professors Jung Kyung-hae at Asan Medical Center, Park Yeon-hee at Samsung Medical Center, Sohn Joo-hyuk at Severance Hospital, and Kim Ji-hyun at Seoul National University Bundang Hospital.

Enhertu hits a home run in HER2-low breast cancer
The DESTINY-Breast04 study is an open-label, multicenter, phase 3 trial that evaluated the efficacy and safety of Enhertu compared to standard chemotherapy in patients with HER2-low HR-positive or negative metastatic breast cancer who had previously been treated.
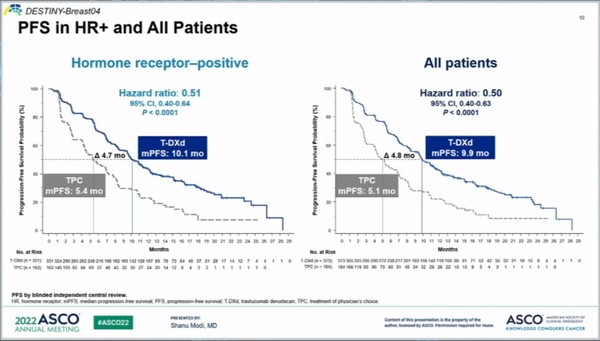
According to the presentation, the median progression-free survival (PFS) of the Enhertu-treated patients, the primary endpoint, was 9.9 months, versus 4.8 months in chemotherapy patients. Enhertu reduced the risk of disease progression or death by 50 percent.
In HR-positive patients who account for 90 percent of the total breast cancer patients, the median PFS of the Enhertu group was 10.2 months vs. 5.4 months of the chemotherapy group. In addition, the drug lowered the risk of disease progression or death by 49 percent.
The median overall survival (OS), the secondary endpoint, was 23.4 months in the Enhertu group, compared with 16.8 months in the chemotherapy group. The drug extended median OS by 6.6 months and reduced the risk of death by 36 percent.
In HR-positive patients, the median OS of the Enhertu treatment group was 23.9 months, while that of the chemotherapy group was 17.5 months. The drug reduced the risk of death by 36 percent.
Also, Enhertu improved objective response rate (ORR) and complete response (CR) significantly compared with conventional chemotherapy.
Across all patients, the ORR was 52.3 percent with Enhertu, compared with 15.3 percent with chemotherapy. Likewise, the CR was 3.5 percent with Enhertu vs. 1.1 percent with chemotherapy.
In HR-positive patients, the ORR of the Enhertu group was 52.6 months, versus 16.3 percent in the chemotherapy group. The CR was 3.6 percent and 0.6 percent, respectively.
Sohn noted that HER2-low breast cancer accounts for about 50-55 percent of metastatic breast cancer.
“T-DXd (Enhertu) proved notable therapeutic effects in HER2-positive patients taking up 15 percent of breast cancer patients and HER-low patients, improving the treatment outcome for nearly 70 percent of breast cancer patients,” he said.
Based on the phase 3 DESTINY-Breast03 study, Enhertu had already become a standard of care for secondary or higher-stage therapy to treat HER2-positive metastatic breast cancer.
According to the DESTINY-Breast 03 results, announced at the annual meeting of the European Society for Medical Oncology in September (ESMO 2021), Enhertu reduced the risk of disease progression and death by 72 percent, compared with Kadcyla (trastuzumab emtansine), the first-generation antibody-drug conjugate (ADC).
Enhertu becomes a gamechanger but a pipe dream for Korean patients
Enhertu changed the paradigm of breast cancer treatment overnight, but Korean breast cancer patients will have to wait for the drug to arrive for quite some time.
“As you can see, T-DXd is already used for HER-2 positive breast cancer in other countries. It is expected to be used for HER2-low patients, too,” professor Sohn said. “But what about Korea? Korean patients can’t use T-DXd even for HER2-positive disease, let alone HER2-low.”
In June last year, the Ministry of Food and Drug Safety granted priority review to Enhertu. However, when the treatment obtains marketing approval remains uncertain.
According to professor Jung, some Korean patients get T-DXd through Korea Orphan & Essential Drug Center (KOEDC), and it takes about 20 million won ($15,931) per treatment cycle. “In Korea, T-DXd is like a pipe dream for breast cancer patients.”
While the ASCO participants cheered for the successful results of the Enhertu study, Jung felt frustrated because Korean doctors and patients do not have access to the drug, she said.
“Korean doctors participated in the DESTINY trials, and they know this drug well, but it is agonizing to see the gap between the reality and study results,” she added.
Professor Kim said hurdles for Enhertu treatment included the government approval and reimbursement review.
“I hope that the government could evaluate the drug’s innovative value from patients' perspective, along with the cost-effectiveness,” she said.
Kim went on to say that it is important for the government to use a flexible reimbursement system. She added that the government does not have to insist on a 5 percent out-of-pocket payment rate for expensive drugs that could burden patients and the national insurance finance.
“Even with these excellent study results, it takes about two to three years, or up to five years, for Korean patients to use that drug,” Kim said. “Enhertu is not even authorized Korea. So considering the time for getting reimbursement, it will take a long time.”
Some might wonder if this Enhertu study outcome deserves a standing ovation, but T-DXd’s PFS was 9.9 months, almost double from 4.8 months of chemotherapy’s PFS, Kim noted.
“This is a huge difference to cancer patients who do not have much time left.”
Professor Sohn said that about 70 percent of metastatic breast cancer patients could benefit from this drug, and 15 percent will get greater help.
T-DXd is an essential drug for Korean breast cancer patients. Thus, the government and pharmaceutical companies should speed up efforts to authorize the drug in Korea.
Professor Park, leader of the breast cancer division at the Korean Cancer Study Group, said the upcoming breast cancer treatment guideline would put Enhertu on the recommended drug list soon.
She emphasized that the government should not delay approval for Enhertu any longer.

