Enhertu, a new breast and stomach cancer treatment, has won domestic approval.
The Ministry of Food and Drug Safety said Monday that it would approve Enhertu Inj. 100 mg (trastuzumab deruxtecan), a breast and stomach cancer treatment, which will be marketed by Daiichi Sankyo Korea.
The domestic approval of Enhertu aims to “treat non-abstractable or metastatic HER2-positive breast cancer patients with two or more administrations of HER2-based treatments, and locally progressive or metastatic HER2-positive gastric or gastroesophageal junction adenocarcinoma patients with two or more administrations of treatments, including anti-HER2 treatment.”
Enhertu is an antibody-drug complex (ADC) co-developed by AstraZeneca and Daiichi Sankyo. Antibodies (trastuzumab) specifically target HER2 expression tumors, while drugs (deruxtecan) have a mechanism to inhibit cell proliferation and induce tumor cell death.
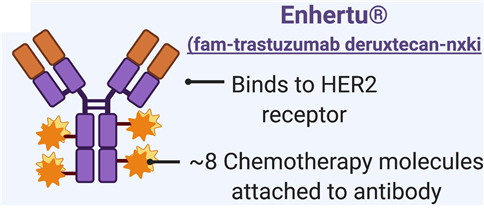
Enhertu has drawn attention by lowering the disease progression and mortality rate by 72 percent compared to the existing ADC treatment, Kadcyla (trastuzumab emtansine), in HER2-positive breast cancer patients, and by 50 percent in HER2-low expression patients for whom there had been no treatments except for anticancer chemotherapies.
It obtained approval from the U.S. Food and Drug Administration for tertiary and secondary treatments of unresectable or metastatic HER2-positive breast cancer patients in December 2019 and May 2021, respectively. However, the drug failed to win the Korean regulator's nod.
Accordingly, Korean patients had to airlift self-treatment drugs at high prices through the Korea Orphan & Essential Drug Center (KOEDC), and concerns have been raised over the lack of cold chain management. In August, a petition called for the official approval of Enhertu, receiving consent from more than 50,000 people.
“Enhertu Inj. 100mg is expected to provide a new treatment opportunity for patients with HER2-expression breast and stomach cancer who have found it difficult to treat with existing treatments,” the ministry said.
Related articles
- ‘If I could use Enhertu, I would sell my house,’ says a breast cancer patient
- US to expand indications for Enhertu – a dream for Korean breast cancer patients
- Father begs new breast cancer drug Enhertu approval for his daughter
- [ASCO 2022] Enhertu gets a standing ovation, but a pie in the sky for Korean patients
- Enhertu likely to replace Kadcyla in HER2-positive breast cancer treatment
- Enhertu provides hope for HER2 breast cancer patients in Korea as secondary standard treatment

