CHICAGO, Ill. -- By Kim Yun-mi/Korea Biomedical Review correspondent -- "The ADAURA study is the first global phase 3 trial to demonstrate a statistically significant and clinically meaningful overall survival (OS) benefit with targeted therapy in patients with resected stage 1B to 3A EGFR-mutated non-small cell lung cancer (NSCLC), further solidifying adjuvant osimertinib therapy as a standard of care for patients with this stage of the disease."
The audience erupted in applause on Thursday in Chicago, local time, as Roy S. Herbst, MD, of Yale Cancer Center, returned to the podium at the plenary session of the annual meeting of the American Society of Clinical Oncology (ASCO 2023) as ADAURA's clinical lead investigator.
AstraZeneca's targeted therapy Tagrisso (osimertinib) was the subject of the plenary session at ASCO 2023, held through Wednesday in Chicago, Ill., based on the phase 3 ADAURA study in patients with early-stage EGFR-mutated NSCLC.
Having been named to the 2020 plenary session after demonstrating a significant disease-free survival (DFS) benefit in the ADAURA study, Tagrisso's innovation has been repeatedly recognized by the most prestigious oncology society, with another plenary session three years later for overall survival (OS) data from the same study.
Adjuvant Tagrisso reduces risk of death by 51% in patients with stages 1B to 3A

Data from the ADAURA study, presented at the plenary session of ASCO 2020, showed that Tagrisso adjuvant therapy improved disease-free survival (DFS) by 83 percent compared to placebo in patients with stage 2-3A, the primary endpoint (hazard ratio 0.17). The secondary primary endpoint analyzed DFS in the entire patient population, including stage 1B patients, with a hazard ratio of 0.21, resulting in a 79 percent reduction in the risk of relapse or death in the entire patient population.
Although the data from the ADAURA study were not fully mature at the time (data maturity 29 percent overall, 11 percent in the Tagrisso arm vs. 46 percent in the placebo arm), the data were presented at ASCO 2020 three years ago after an independent data monitoring committee recommended early data release based on the remarkable results.
An updated DFS analysis, published in January of this year in the American Society of Clinical Oncology's journal JCO (data maturity 45 percent overall, 28 percent in the Tagrisso arm vs. 62 percent in the placebo arm), showed that adjuvant Tagrisso reduced the risk of recurrence or death by 73 percent in the overall patient population (stages 1B through 3A) (hazard ratio 0.27), with a CNS DFS hazard ratio of 0.24 for Tagrisso compared to placebo in stages 2 through 3A, reducing the risk of brain metastasis recurrence or death by 76 percent.
The question was whether this improvement in DFS with adjuvant Tagrisso translated into longer patient survival, and the answer was revealed at ASCO 2023.
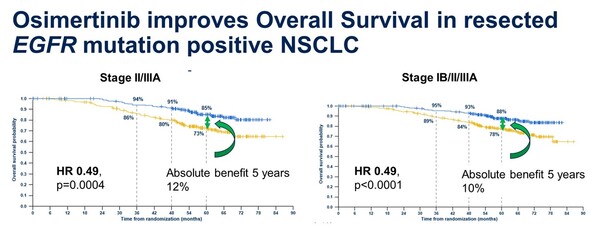
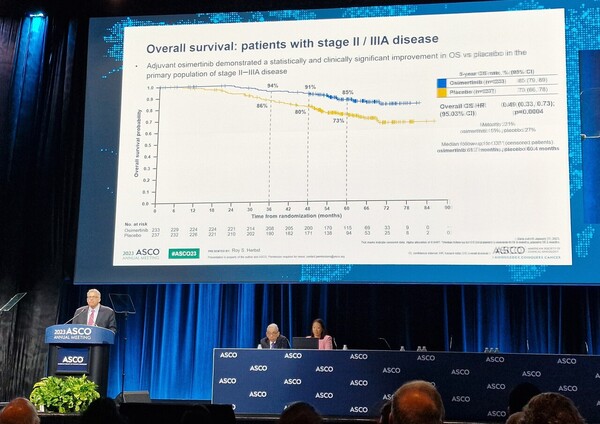
Tagrisso adjuvant therapy reduced the risk of death by 51 percent compared to placebo in patients with stage 2-3A, the primary endpoint (hazard ratio 0.49). The five-year overall survival rate in the Tagrisso arm was 85 percent compared to 73 percent in the placebo arm, a 12 percent increase in absolute survival.
This was confirmed across all patient populations, including those with 1B. In all patients, the five-year overall survival rate was 88 percent in the Tagrisso arm compared to 78 percent in the placebo arm, representing a 51 percent reduction in the risk of death (hazard ratio 0.49) and a 10 percent higher absolute survival rate.
This OS benefit of Tagrisso adjuvant therapy was consistent regardless of patient race, stage, EGFR mutation type, or prior chemotherapy.
Notably, the study showed that in patients with advanced disease, most patients received an EGFR TKI as follow-up treatment, and despite a higher rate of EGFR TKI follow-up with Tagrisso in the placebo arm, the study achieved OS improvement (EGFR TKI follow-up rate: 76 percent in the Tagrisso arm vs. 88 percent in the placebo arm).
The incidence of grade 3 or higher adverse events was 23 percent in the Tagrisso arm and 14 percent in the placebo arm. No treatment-related deaths were reported in either arm.
ADAURA opens door to precision medicine in early lung cancer

Benjamin Solomon, PhD, of the Peter MacCallum Cancer Centre and The University of Melbourne
"The ADAURA study is the first phase 3 trial to demonstrate an overall survival benefit of a targeted therapy in the adjuvant setting for NSCLC," said Dr. Benjamin J. Solomon, Peter MacCallum Cancer Centre, Australia, who took the podium following Dr. Herbst's presentation. "By expanding the use of lung cancer targeted therapies from advanced disease to earlier stages, the ADAURA study is a groundbreaking trial that opens a new frontier for precision medicine in early-stage NSCLC."
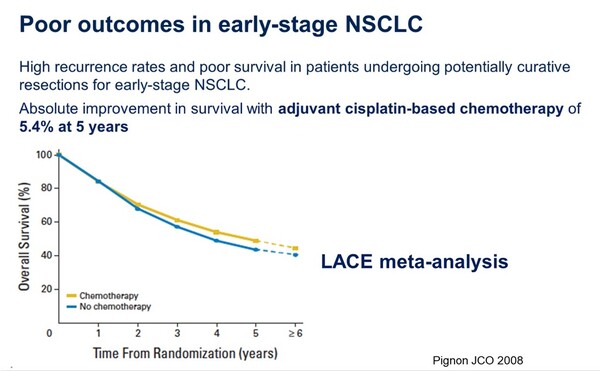
"Even with curative resection, patients with early-stage NSCLC have high recurrence rates and poor survival," said Dr. Solomon, explaining the poor prognosis of early-stage NSCLC.
Dr. Solomon emphasized the need for mandatory EGFR mutation testing for all early-stage NSCLC patients and mandatory Tagrisso adjuvant therapy for resected stage 1B-3A (now 2A-3A) patients.
"Global support for testing and treatment access/insurance is needed to ensure that global access gaps and inequalities in effective cancer treatment are not exacerbated," Dr. Solomon added.
ADAURA delivers breakthrough for early NSCLC at high risk of recurrence

Professor Kim Hye-ryun of Oncology at Severance Hospital, who serves as a director of health insurance at the Korean Association for Lung Cancer, also recognized the value of the ADAURA study at ASCO 2023.
Tagrisso is the only targeted therapy that has demonstrated an overall survival benefit as well as a prolongation of disease-free survival in patients with early-stage NSCLC who are at high risk of recurrence, she said.
"Lung cancer is a highly recurrent cancer, even when detected and treated at an early stage," said Professor Kim. "The recurrence rate varies by stage, with 40 percent of patients in stage 1, 45 percent in stage 1B, 62 percent in stage 2, and 76 percent in stage 3 experiencing recurrence."
Once you have a recurrence, you're in a stage where you can't be cured, she noted.
"Speaking from the perspective of someone who sees recurrences all the time in the hospital, if you can increase disease-free survival while continuing to take medication after surgery, that's significant in itself."
For example, assuming that overall survival is the same, there is a big difference between patients with longer cancer-free survival and those with shorter cancer-free survival, Kim explained.
"Extended disease-free survival has many benefits for patients, including their daily lives," she said. “And osimertinib adjuvant therapy has shown dramatic results in extending not only disease-free survival but also overall survival."
Professor Kim also highlighted the benefits of Tagrisso adjuvant therapy in patients with brain metastases at relapse.
"In patients with EGFR mutations, the percentage of patients who develop brain metastases at the advanced stage of relapse is about 30 percent," she said. "With each subsequent line of treatment, the percentage of brain metastases increases by 20-30 percent, so by the time the patient dies, most of them have brain metastases."
However, as long as Tagrisso adjuvant therapy remains non-reimbursable in Korea, the difficulties in the clinic will increase, she predicted.
From a patient's perspective, it is not easy to use osimertinib, which costs 6 million won per month in non-payment, Professor Kim noted.
“I explain osimertinib adjuvant therapy in stage 3 patients with the highest risk of recurrence and present treatment options, but now that we have proven the overall survival benefit, we are obliged to explain it to early patients, so difficulties in the clinical field are expected."
Related articles
- [Photo News] ASCO returns to full strength, with Korean firms actively engaged
- AZ’s Tagrisso broadens treatment options for EGFR-mutant lung cancer
- Tagrisso vs. Leclaza – let the best drug win
- Calls mount to expand NSCLC treatment Tagrisso’s insurance benefits
- ‘I’m living a second life after taking Tagrisso for 3 months’
- Korean cancer researchers present 139 studies at ASCO 2023
- Aptamer Sciences' non-reimbursed early detection lung cancer kits available at tertiary hospitals
- Why doctors should use immunotherapy for early lung cancer patients
- ‘Immunotherapies to serve as cornerstone in stage 4 gastric cancer treatment’
- AstraZeneca, SK Chemicals mark 1st production of diabetes combo
- [Reporter’s notebook] What the government misses by not reimbursing Tagrisso
- AZ’s Tagrisso moves closer to expanded reimbursement as 1st-line cure of lung cancer
- [Interview] Tagrisso offers a new option for 1st-line treatment of stage 4 lung cancer

