Korean researchers drew attention at the American Society of Clinical Oncology annual meeting, ASCO 2023, by presenting a variety of therapeutic strategies, including immunotherapies, which will contribute to improving the treatment landscape for significant and rare cancers.
The Korea Cancer Study Group (KCSG) said Korean researchers made 139 oral and poster presentations as first authors, including research on overcoming immunotherapy resistance and the efficacy of intracranial targeted anticancer drugs for brain metastases, at ASCO 2023 in Chicago, Ill., from the last Friday to Tuesday.
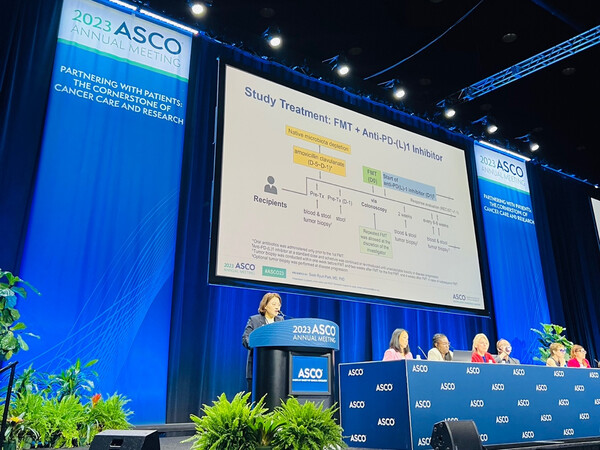
Fecal transplants show possibility of overcoming immunotherapy resistance
Immunotherapy has become the standard treatment demonstrating excellent outcomes in many different types of cancer, but it has the limitation of developing resistance in most patients.
Recently, attempts have been made to increase immunotherapy’s effectiveness using the gut microbiome known to influence the body's immune system.
At the ASCO 2023 Clinical Science Symposium, Dr. Park Sook-ryun, a professor of medical oncology at Asan Medical Center in Seoul, presented the results of a clinical study where feces from patients who responded well to immunotherapy were transplanted into immunotherapy-resistant patients.
In the study, 13 patients with solid tumors (liver, stomach, and esophagus) were transplanted with feces. The size of cancer in one patient was significantly reduced, and those of five others were stabilized, confirming the clinical effectiveness and anticancer immune activation of fecal transplantation.
"This study is significant because it clinically demonstrates the feasibility of using gut microbes to overcome immuno-oncology resistance in solid tumors,” Professor Park said. “It has opened the door to developing new anticancer drugs based on gut microbes."
Immunotherapy ‘pembrolizumab’ proves efficacy in multiple combinations
Because people with biliary tract cancer tend to have poorer systemic status than those with other cancers, it is essential to maintain survival and quality of life when adding chemotherapy.
Professor Yoo Chang-hoon of the Department of Medical Oncology at Asan Medical Center orally presented the results of an evaluation and analysis of patients' quality of life when pembrolizumab (Keytruda) was added to standard anticancer therapy in people with inoperable, locally progressive, or metastatic biliary tract cancer.
A sub-analysis of the phase 3 clinical trial comparing the three-drug combination therapy of gemcitabine, cisplatin, and pembrolizumab to the gemcitabine plus cisplatin plus placebo showed that the addition of pembrolizumab resulted in a similar quality of life for patients to the placebo group.
"This analysis confirms that the gemcitabine plus cisplatin plus pembrolizumab regimen improves overall survival and is safe with a low impact on quality of life,” Professor Yoo said. “It suggests that the three-drug regimen, including pembrolizumab, should be the standard primary treatment for patients with biliary tract cancer."
Dr. Rha Sun-young, chair of the Gastric Cancer Section of the KCSG and professor of the Department of Oncology at Yonsei Cancer Center, presented a poster at ASCO 2023 on the results of a subgroup analysis based on PD-L1 expression from a multicenter, multinational, double-blind, phase 3 study.
It confirmed the efficacy of adding the immunotherapy pembrolizumab to a two-drug regimen of cytotoxic antitumor agents in the first-line treatment for HER2-negative, advanced, and metastatic gastric cancer.
The analysis showed improved survival and response rates with pembrolizumab in both groups with PD-L1 composite positive score (CPS) of 1 or higher and 10 or higher.
"This study provides important support for establishing immuno-oncology combination therapy as a standard of care in the first-line treatment of advanced or metastatic gastric cancer," Professor Rha said. "Further studies are warranted to identify the patient group that will benefit the most.”
Homegrown targeted agent lazertinib rises as treatment strategy for brain metastases
Dr. Hong Min-hee, a medical oncologist at Yonsei University Cancer Center, presented a poster on the results of a multicenter, phase 2 study evaluating the intracranial efficacy of the third-generation EGFR targeted therapy, lazertinib (Lexarza), in patients who experienced disease progression in the central nervous system after using first- or second-generation EGFR targeted therapies.
Researchers enrolled 40 patients at six centers, with the primary endpoint of intracranial response and the secondary endpoint of intracranial progression-free survival (PFS), the intracranial response rate in T790M mutation-negative patients, overall response rate, overall disease control rate, and safety.
The study showed that patients taking lazertinib met the primary endpoint and had excellent intracranial response rates. It was the same even in T790M-negative patients, who comprised the majority of study participants, and in patients with leptomeningeal metastases, which show a very poor prognosis.
"Lazertinib showed significant CNS activity in patients with brain metastases who failed conventional EGFR-targeted therapies, regardless of T790M mutation status,” Professor Hong said. “It suggests that using lazertinib as an alternative to local therapy may be a valid treatment strategy for patients with EGFR-mutated NSCLC who have developed CNS metastases after first- and second-generation targeted therapies."
Related articles
- ‘Palliative care neglected amid a flood of expensive cancer drugs’
- [ASCO 2023] Tagrisso shows ‘breakthrough’ benefit in early lung cancer
- [Photo News] ASCO returns to full strength, with Korean firms actively engaged
- Why doctors should use immunotherapy for early lung cancer patients
- ‘Immunotherapies to serve as cornerstone in stage 4 gastric cancer treatment’

