Coreline Soft, a medical AI company, said Friday that AVIEW PE, which detects pulmonary embolism, has been designated an innovative medical device under the Ministry of Food and Drug Safety's Integrated Innovation Designation No. 68.
The designation allows Coreline Soft to supply AVIEW PE to clinics on a non- or selective reimbursement basis for at least three years. During this time, the company will establish clinical evidence, including efficacy, and the device will undergo another New Medical Device Evaluation. If it passes this process, the product will be eligible for reimbursement.
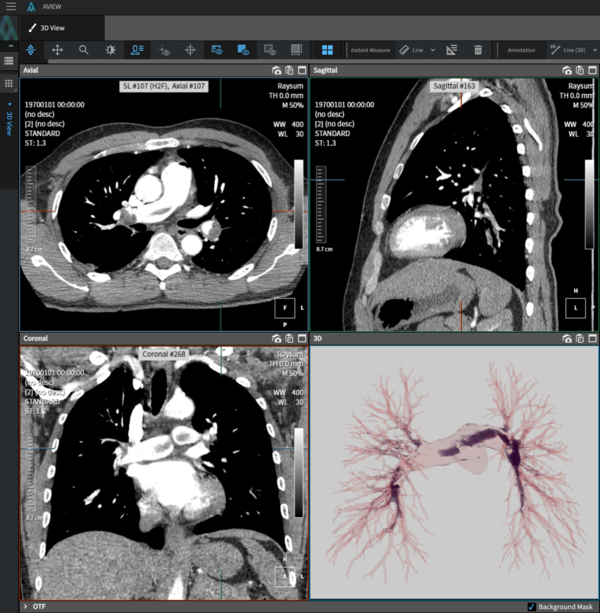
AVIEW PE (Pulmonary Embolism) is a software that automatically detects pulmonary embolism and assists in diagnosis based on artificial intelligence. It has been designated as the first innovative medical device in Korea in the pulmonary embolism diagnostic aid category
With the designation of AVIEW PE as an innovative medical device, Coreline Soft has the largest number of innovative integrated medical devices in Korea, including cerebral hemorrhage and aortic dissection diagnostic aids, previously designated as innovative medical devices.
"With this innovative medical device designation, we expect to distribute AVIEW Aorta and AVIEW PE rapidly to the emergency care area, helping to improve the prognosis of patients by assisting in the rapid and accurate diagnosis of severe patients who come to the emergency room with chest pain or breathing difficulties," said Park Joon-min, Coreline Soft’s chief product officer and a former emergency medicine specialist. "We will actively develop a step-by-step marketing strategy for non-reimbursement entry and fee confirmation by perfecting the medical imaging platform optimized for the emergency room treatment environment."
In January, Coreline Soft entered the non- or selective reimbursement market with AVIEW NeuroCAD, an AI-based brain hemorrhage diagnostic aid. AVIEW NeuroCAD automatically analyzes the amount of bleeding in a patient's brain CT image, enabling doctors to read the image and make diagnostic and treatment decisions within a limited time.
In February, AVIEW Aorta, a cardiovascular image detection and diagnosis aid software, was designated an innovative medical device. It is the first product in Korea designated an aortic dissection diagnostic aid and was awarded a Grade 3 status in recognition of its technology and stability.
Related articles
- AI tech co-developed by Coreline Soft, AMC enhances sarcopenia diagnosis
- Coreline Soft launches AI solution 'AVIEW CHEST'
- Coreline Soft opens European door by teaming up with global hospitals
- Coreline Soft to present 2 thoracic solutions and research abstracts at ATS 2024
- Coreline Soft enters UK lung cancer screening teleradiology market
- Medical AI companies begin to supply homegrown solutions
- Coreline Soft begins non-reimbursed billing for AVIEW NeuroCAD brain CT analysis solution
- Coreline Soft to showcase advanced AI solutions at SCCT 2024 in Washington DC
- Coreline Soft scores nod for AI-powered pulmonary embolism detection software
- Coreline Soft to showcase AI solutions at NASCI 2024, accelerating US entry

