Coreline Soft said Friday that it would participate in the North American Society for Cardiovascular Imaging (NASCI) in Boston, Mass., from Saturday to next Tuesday.
NASCI attracts leading experts in heart disease from around the world to share information and clinical cases using the latest devices.
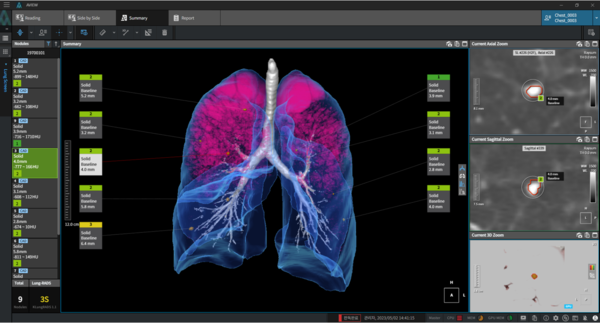
At the meeting, Coreline Soft will demonstrate AIVEW LCS Plus, which simultaneously analyzes the “Big Three” indications of lung disease -- lung cancer, emphysema, and coronary artery disease. AIVEW LCS Plus can detect multiple conditions in a single chest CT scan, enabling early detection of many diseases, not just lung cancer. It has already been used as a solution for various global lung cancer screening projects organized by the European Union, Germany, Italy, and others.
AVIEW CAC, an artificial intelligence cardiovascular diagnostic solution, will also be demonstrated. It automatically analyzes and quantifies image data obtained from computed tomography (CT).
According to Coreline Soft, a performance evaluation study was conducted on 130 images, including 87 CT images with up to five nodules and 43 images without nodules, using AVIEW CAC. The study found that it took an average of 585 minutes to read the images without AI, but with AI, the average time was reduced by nearly 60 percent to 235 minutes. The results were presented at the Radiological Society of North America (RSNA 2023) in the United States last year.
Last year, Coreline Soft also received medical device certification from the U.S. Food and Drug Administration (FDA) for its AI-based lung nodule detection solution (AVIEW Lung Nodule CAD). It was the first Korean product to receive FDA certification for an AI-based lung nodule detection solution and the fifth worldwide.
Coreline Soft has supplied key products to UMass Memorial Medical Center, a cancer treatment center in Worcester, Mass., since June. In 2023, UMass was ranked among the top five hospitals in Massachusetts by U.S. News and World Report and provides all levels of healthcare, from primary to quaternary care.
UMass has adopted Coreline Soft's three main products—AVIEW LCS, AVIEW COPD, and AVIEW CAC—and plans to conduct an in-depth lung cancer screening study based on AVIEW LCS Viewer to demonstrate its real-world clinical applicability. Ultimately, the two companies will set a new standard for CT reading with AI and collaborate through joint research and educational programs.
“Significantly, UMass is the first to adopt our technology, including AVIEW LCS, into its clinical workflow and use it in real-world clinical applications through the LCS Dedicated Viewer,” said Lee Jae-heon, president of Coreline Soft North America. “We look forward to setting a new standard in lung cancer screening and treatment through this collaboration, and we will continue to work together to contribute to a better healthcare experience.”
Related articles
- Coreline Soft partners with German hospital, Scottish AI platform to accelerate medical AI expansion
- Coreline Soft scores nod for AI-powered pulmonary embolism detection software
- Coreline Soft to showcase advanced AI solutions at SCCT 2024 in Washington DC
- Coreline Soft’s AVIEW PE labeled as innovative medical device
- AI tech co-developed by Coreline Soft, AMC enhances sarcopenia diagnosis
- Coreline Soft launches AI solution 'AVIEW CHEST'
- TB society urges public not to ignore early symptoms of pulmonary diseases
- Coreline Soft to showcase AVIEW COPD solution at GOLD International COPD Conference
- Coreline Soft expands US presence with AVIEW software adoption by Temple Lung Center
- [RSNA 2024] Coreline Soft expands US presence with AI-powered imaging solutions
- Coreline Soft lands FDA clearance for upgraded AI coronary calcium analysis software
- Coreline Soft doubles AI teleradiology contract price in European deal

