ATsens, a Korean bio-signal-based healthcare platform company, said it has signed a major supply agreement with a key U.S. distributor for its wearable electrocardiogram (ECG) monitoring device AT-Patch and its ECG data analysis software AT-Report.
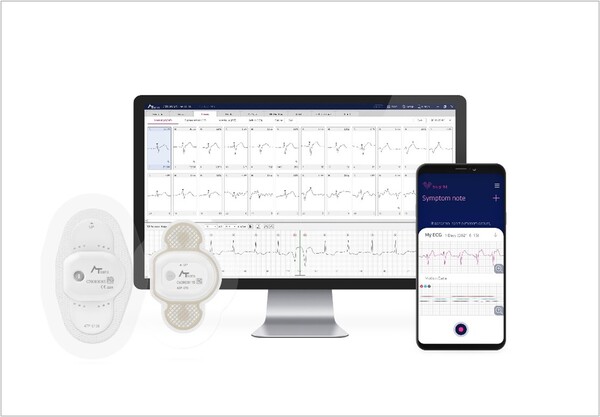
The deal will allow the company to supply its products for three years and represents the first large-scale U.S. supply contract for a Korean-made wearable ECG monitoring device.
However, the company did not unveil the name of its partner, citing contractual reasons.
Under the agreement, ATsens will begin supplying about 10 billion won ($7.2 million) worth of devices, equivalent to 100,000 units, in 2026 to the distributor, which has strong networks in New York, Florida, and Arizona, before expanding to a cumulative 50 billion won (450,000 units) over three years.
ATsens is also in talks with larger distributors capable of nationwide coverage, anticipating exponential growth in export volumes.
Since its inception, ATsens has focused on overseas expansion, securing regulatory approvals from the U.S. FDA, Europe’s CE, Japan’s PMDA, and the U.K.’s MHRA.
It has already signed contracts in over 30 countries and commenced shipments to more than 10 markets. Notably, the company penetrated Japan’s highly competitive arrhythmia market in 2022 and now supplies AT-Patch to over 150 major hospitals.
AT-Patch is one of only two products, alongside iRhythm Technologies’ Zio Patch, that offer continuous ECG monitoring for up to 14 days without battery replacement or charging.
In the U.S. long-term cardiac monitoring (LTCM) market, which iRhythm currently dominates with a 70 percent share, ATsens aims to differentiate itself with its integrated AT-Patch and AT-Report solution.
Unlike iRhythm’s model, which requires data analysis through its centralized independent diagnostic testing facility (IDTF), ATsens’ AT-Report software allows local hospitals to analyze ECG data on-site within days, significantly reducing the three to four week turnaround typically associated with iRhythm.
The company expects that the faster analysis will enable quicker clinical intervention and enhances diagnostic efficiency for healthcare providers.
“AT-Patch and AT-Report are differentiated solutions that address unmet needs in the U.S. ECG monitoring market,” ATsens CEO Jeong Jong-ook said. “By establishing a robust U.S. supply chain through this contract, we will rapidly scale our market share.”
Leveraging its FDA-cleared technology and commercialization experience across Europe, Japan, and the Middle East, the company aims to deliver real value to both patients and healthcare providers in the U.S. and accelerate its growth as a global healthcare platform company, Jeong added.
Related articles
- Korean radiologists question whether AI diagnostics are ready for clinical reality
- ATsens’s Himalayan arrhythmia risk study using AT-Patch published in JAMA Cardiology
- ATsens, Huinno vie for piece of global ECG patch monitoring market
- ATsens takes world's first arrhythmia clinical study to Mount Everest
- ATsens secures coverage for real-time monitoring system for hospitalized patients

