ATsens, a wearable medical device company, announced Thursday that its AT-Monitoring system for real-time inpatient tracking has qualified for insurance benefits under the “Remote Heart Rate Monitoring (EX871)” procedure, as designated by the Health Insurance Review and Assessment Service (HIRA) in August.
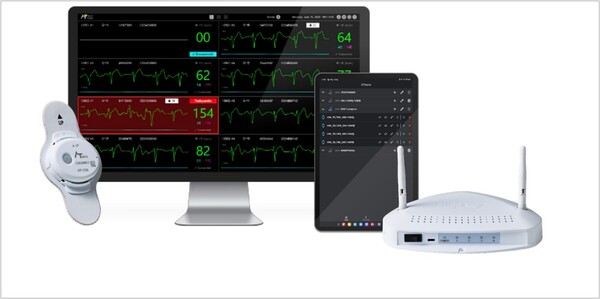
AT-Monitoring is a Class 2 medical device. As a "patient central monitoring device," it provides real-time monitoring of hospitalized patients' electrocardiograms (ECG), which record the heart’s electrical activity, and falls. The wearable Holter ECG device AT-Patch continuously monitors and collects biometric signals, then transmits them to a dedicated monitoring viewer with arrhythmia alarm functionality (alerts for irregular heart rhythms). This setup enables medical staff to instantly review patient status at the bedside and respond immediately to any abnormal signs.
Patients using the AT-Patch with AT-Monitoring do not replace batteries or electrodes during hospitalization. The device's IP57 waterproofing lets patients shower and remain active, while continuously monitoring ECG. This design enhances patient comfort and guarantees reliable, uninterrupted ECG monitoring.
ATsens plans to gradually add additional measurement items to AT-Monitoring, including transcutaneous oxygen saturation (E7230) and 24-hour ambulatory blood pressure (E6548).
ATSsens reported that after securing insurance reimbursement, it contracted with over 10 tertiary and general hospitals, and some key national university hospitals confirmed full-bed implementation.
“AT-Monitoring is a solution that enhances patient safety and hospital operational efficiency. It also minimizes adoption barriers through reasonable initial setup costs,” Atsens CEO Jeong Jongook said. “With this insurance reimbursement approval, we are pursuing contracts with major medical institutions for about 4,000 beds by the end of 2025. We plan to keep expanding the domestic market, targeting installation of 25,000 beds by 2026.”
ATsens now holds four major international regulatory approvals for its wearable ECG diagnostic solution, including the U.S. FDA, European CE, Japan's PMDA, and the U.K.'s MHRA.

