Samjin Pharmaceutical announced on Wednesday that Wellysis has obtained U.S. FDA approval for S-Patch Ex, a wearable electrocardiogram (ECG) monitor.
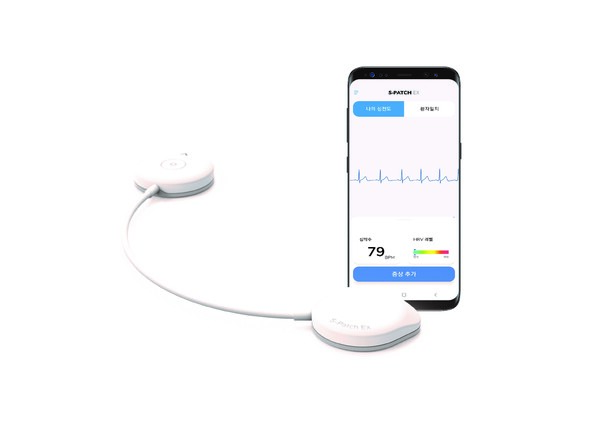
S-Patch Ex has been developed by Wellysis, a digital healthcare startup that spun off from Samsung SDS in 2019. In the Korean market, Samjin Pharmaceutical is responsible for selling this ECG device.
S-Patch Ex is a lightweight and compact device with a thickness of 6 mm and a weight of 9 g. It is equipped with waterproof and dustproof features, boasting an IP55 rating, ensuring durability in various conditions. This device plays a crucial role in diagnosing arrhythmia and heart disease.
Korean physicians have been incorporating this device into their practice for diagnosing arrhythmias. They appreciate its high wearing comfort, its ability to provide precise diagnostic results, and the clarity of the generated ECG result reports, Samjin said.
S-Patch Ex has obtained registrations as a medical device from the European CE, Australian TGA, New Zealand MEDSAFE, and Korea’s Ministry of Food and Drug Safety. The company has made the device available in 14 countries.

