VUNO said VUNO Med-DeepBrain, its artificial intelligence (AI)-based brain imaging analysis medical device, has received 510k clearance from the U.S. FDA.
This marks the first product developed by the company to receive FDA approval.
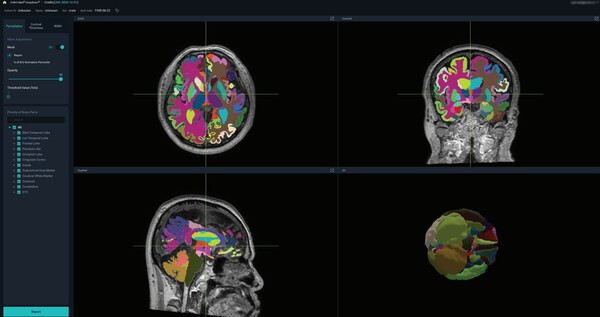
The device analyzes brain magnetic resonance imaging (MRI) images based on deep learning, parcellates the brain into more than 100 brain regions, and provides quantified information on the degree of atrophy in each region within one minute.
"This helps doctors diagnose dementia caused by major degenerative brain diseases such as Alzheimer's disease and vascular dementia," the company said. "It can also contribute to pre-screening patients who are likely to progress from mild cognitive impairment to dementia."
Having secured FDA certification, the company intends to bolster its sales and marketing efforts targeted at local healthcare organizations through its U.S. subsidiary, enabling a swift market entry.
Furthermore, the company intends to enhance its collaborations with globally recognized pharmaceutical firms seeking AI-based brain MRI quantification technology, given the anticipated rapid expansion of the early dementia diagnosis market following the FDA's approval of Alzheimer's disease drugs this year.
VUNO had previously confirmed the potential of VUNO Med-DeepBrain for early diagnosis of Alzheimer's disease through clinical studies.
In July, during the Alzheimer's Association International Conference (AAIC 2023), a clinical study was presented, showcasing the device's capacity to potentially diagnose Alzheimer's disease in individuals experiencing subjective cognitive decline (SCD). This represents an early stage of the disease, occurring prior to the manifestation of full-fledged dementia symptoms in patients.
The company also said that brain MRI also has the advantage of being relatively low-cost and accessible compared to PET (positron emission tomography) scans, which are used to test for the presence of amyloid, one of the diagnostic criteria for Alzheimer's disease.
"VUNO Med-DeepBrain is an AI medical device that has demonstrated excellent clinical efficacy through a number of global conferences and journals, and we expect the device will become the cornerstone of VUNO's expansion into the U.S. market," VUNO CEO Lee Ye-ha said. "With the recent emergence of next-generation dementia therapies and increasing demand for early diagnosis, we will strengthen our sales efforts to rapidly expand in the U.S. market."
Related articles
- Leading Korean AI medical imaging companies showcase key products at KCR 2023
- 'VUNO Med-DeepCARS outperforms traditional methods in cardiac arrest prediction'
- VUNO’s cardiac arrest-predicting device now available for children under 19
- VUNO shares surge after FDA innovative medical device designation
- VUNO to co-develop medical content with Weknew
- VUNO to disclose study results of AI-based ECG analysis software at AHA 2023
- VUNO’s Q3 sales surge with success of AI-based cardiac arrest management system
- VUNO, Readycure sign MOU to develop AI-based dementia treatment medical devices

