Huons Meditech said it has obtained import approval for TULSA-PRO, a prostate cancer and benign prostatic hyperplasia treatment device, developed by Profound, a Canadian medical device company.
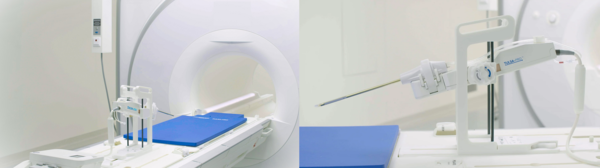
TULSA-PRO is a treatment device based on ultrasound technology. The technology propagates through biological tissue, transforms into heat, and is absorbed by the tissue. The heat energy generated causes necrosis of the lesion tissue.
TULSA-PRO represents a new approach to prostate cancer and hypertrophy treatment, differentiated from the known HIFU (High Intensity Focused Ultrasound) method. While HIFU delivers focused ultrasound energy externally through the rectum, TULSA-PRO directly delivers energy inside the prostate via the urethra.
According to Huons Meditech, the treatment method has the advantage of delivering energy precisely to the target location while minimizing the impact on surrounding tissues.
It also allows physicians to confirm the location and size of treatment lesions based on MRI imaging and enables precise treatment through robotics control, while real-time monitoring during the treatment process minimizes potential side effects like incontinence and erectile dysfunction, common in surgical procedures.
With the launch of TULSA-PRO, Huons Meditech is expanding its presence in the urology market with devices like the extracorporeal shock wave lithotripter (ESWL), DuroFill (temporary penile enlargement filler), and IMPO88 (erectile dysfunction treatment device).

