Pfizer’s Vyndamax (tafamidis), a treatment for transthyretin amyloid cardiomyopathy (ATTR-CM), got in the European Society of Cardiology’s (ESC) list of recommended drugs for heart failure.
However, observers said it is uncertain whether the treatment would be reimbursable in Korea because of its high price.
In late August, the ESC released the updated treatment guidelines for heart failure, newly suggesting treatment strategies for heart failure patients with comorbidities.
In the past, the ESC had categorized treatment strategies for comorbidities by each disease. In the new version, the guidelines applied upper categories into “cardiovascular comorbidities,” “non-cardiovascular comorbidities,” and “special conditions.”
The ESC guidelines newly included amyloid cardiomyopathy in the “special conditions” category, providing diagnosis and treatment methods for heart failure patients with amyloid cardiomyopathy.
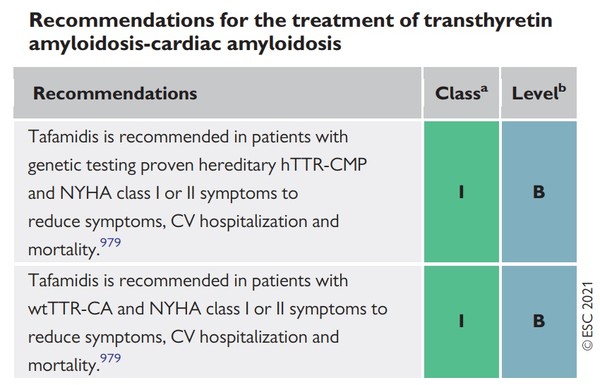
According to the guidelines, amyloid cardiomyopathy (CA) is largely divided into two forms -- light chain immunoglobulin (AL) and transthyretin (ATTR) amyloidosis. In addition, ATTR-CA includes the wild-type (more than 90 percent of cases) and the hereditary/variant type (less than 10 percent of cases).
The ESC guidelines estimated that 6 percent to 16 percent of all patients with unexplained left ventricular hypertrophy (LVH) or HF with preserved ejection fraction (HFpEF) at hospitalization or severe aortic stenosis undergoing aortic valve replacement, aged above 65 years, may have wild-type transthyretin cardiac amyloidosis (wtTTR-CA).
Thus, patients aged 65 or more with heart failure along with a left ventricular wall thickness exceeding 12 mm at echocardiography should be suspected of CA, the guidelines said.
The ESC recommended using tafamidis (Vyndamax) as grade 1B to reduce cardiovascular hospitalization and mortality in patients with grade 1-2 heart failure (NYHA class I-II) with wild-type and hereditary transthyretin CA.
As Vyndamax solidified its effect on ATTR-CM with the ESC’s recommendation, Pfizer reportedly applied for the Health Insurance Review and Assessment Service’s reimbursement.
Earlier, Pfizer failed to make Vyndamax designated as an essential medicine.
This time, the company challenges to win reimbursement using the risk-sharing agreement (RSA) because the government expanded the scope of RSA application from anticancer and orphan drugs to treatments for serious diseases.
However, it will be unclear whether Vyndamax will be eligible for an RSA. In addition, some critics said that Vyndamax is too expensive -- costing over 200 million won ($170,853) annually in the U.S. As a result, the number of target patients might be larger than expected.
In a paper published in the JAMA Cardiology in June, U.S. physicians pointed out that Vyndamax exceeded the cost-effectiveness threshold due to its hefty price tag almost equivalent to those of orphan drugs. They also said ATTR-CM was not a rare disease anymore.
The ESC guidelines also said that ATTR-CM was underestimated as a cause for heart failure. The guidelines emphasized that current diagnostic tests offered 85-100 percent sensitivity and specificity to detect wild-type and hereditary CA patients.
This signals that a larger-than-expected number of patients could be eligible for Vyndamax treatment.
When the size of the patient group has not been identified clearly, granting reimbursement for a drug that costs 2 million won a year could pose a threat to the financial stability of the health insurance program, observers said.

