
The treatment of biliary tract cancer, one of the cancers with a poor prognosis, is changing rapidly. Immunotherapies and targeted anticancer drugs have been developed in a rush. However, none of them have been covered by health insurance in Korea, and patients and medical workers complain that the country remains a "barren land for new drugs.”
According to data from the Korea Central Cancer Registry released in 2023, biliary tract cancer is the ninth most common cancer in Korea, accounting for 2.7 percent of all cancer cases.
Despite its low prevalence, the cancer is difficult to diagnose early and has a poor prognosis on average due to its ability to metastasize to surrounding organs and lymph nodes. The five-year relative survival rate (2017-2021) in Korea was reported to be 28.9 percent for both men and women. For patients with distant metastases, the five-year relative survival rate drops to 3.2 percent, making it a dreaded cancer.
However, recent advances in biliary tract cancer treatment have been remarkable. Immuno-oncology and targeted anticancer drugs have been developed for treating locally progressive, advanced, or metastatic patients unable to undergo radical resection, making precision medicine possible unlike in the past.
In the past, chemotherapy, such as gemcitabine/cisplatin (GemCis) and FOLFOX, were the mainstay of first and second-line treatment. Now, however, targeted therapy based on genetic mutations using anti-PD-(L)1 immuno-oncology drugs for first-line treatment and IDH1-FGFR2-BRAF-HER2 inhibitors for second-line treatment has become the standard therapy.
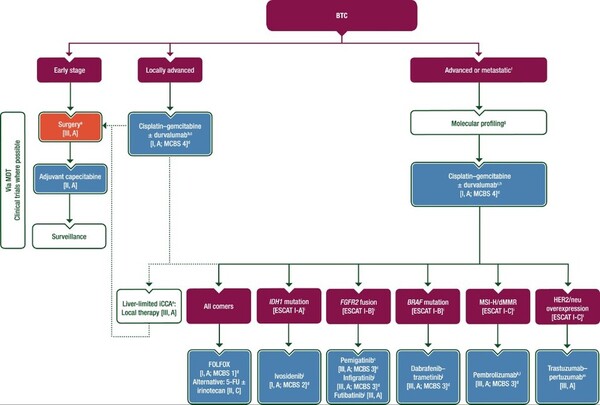
The European Society for Medical Oncology (ESMO) guidelines recommend the combination of durvalumab (Imfinzi in the product name) + GemCis as the standard of care for first-line treatment in patients diagnosed with locally advanced, advanced, or metastatic biliary tract cancer and a combination of targeted and immuno-oncology agents depending on the gene mutation for second-line treatment in patients with advanced disease (refer to the chart). For IDH1 mutations, ivosidenib monotherapy; for FGFR2 fusions, pemigatinib (Pemazyre) or futibatinib monotherapy, for BRAF mutations, dabrafenib (Rafinlar) + trametinib (Meqsel) combination therapy, for MSI-H/dMMR, pembrolizumab (Keytruda) monotherapy, and for HER2/nue overexpression, trastuzumab + pertuzumab (Perjeta) combination therapy are recommended.
However, none of these new drugs are available for reimbursement in Korea. Besides ivosidenib and futibatinib, which have not yet been introduced in Korea, the drugs introduced and used for other cancers are also not covered by insurance. They can only be used through prior application, making them significantly less accessible.
Last year, Handok applied for insurance coverage upon Pemazyre's approval but failed to set coverage standards after a review by the Health Insurance Review and Assessment Service's Cancer Disease Review Committee (CDRC). In late 2022, AstraZeneca also won approval for the Imfinzi + GemCis combination and applied for benefits the following year, but it, too, was stalled at the CDRC.
The only consolation was that, in the past, if patients wanted to use Imfinzi at their own expense, they had to pay for GemCis in combination with Imfinzi, which increased the burden on patients. Last November, however, CDRC approved GemCis therapy in combination with Imfinzi as a “partial payment (5/100),” allowing patients to use Imfinzi at 100 percent out-of-pocket.
In addition, MSD Korea submitted a lump-sum reimbursement application for 13 indications for Keytruda last year, including "metastatic solid tumors with MSI-H/dMMR who have received prior therapy and have no alternative treatment options," which is currently being reviewed by CDRC, but biliary tract cancer was not included in it.
As things stand now, biliary tract cancer patients in Korea who are unable to use the treatment for reasons such as “disapproval,” “lack of coverage,” or “prohibitively expensive” are getting farther away from optimal treatment, according to patients and medical workers.

