Daewoong Pharmaceutical said it has confirmed the efficacy of its “Foistar” tablets in improving the inflammation in Covid-19 patients.
Researchers compared the clinical efficacy and safety by comparing seven patients that took Foistar tablets (camostat mesylate) with 22 patients who took Kaletra tablets (Lopinavir/ritonavir). Kaletra is an HIV treatment most frequently used to treat mild coronavirus cases.

They analyzed the results from August to September, using the C-reactive protein (CRP) test for comparative analysis. It is the most sensitive response indicator for inflammatory symptoms. The higher the inflammation in the liver, the higher the CRP level.
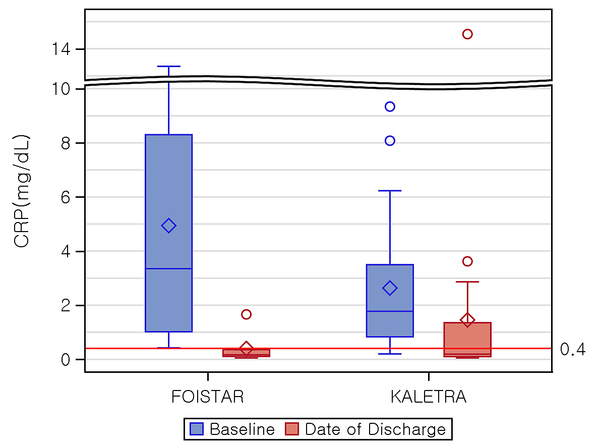
Six out of the seven patients administered with Foistar tabs, or 85.71 percent, were adjusted to normal levels. In comparison, among patients who took Kaletra, 11 out of 18 patients who showed abnormal CRP figures at the time of hospitalization, or 61.1 percent, were adjusted to normal levels. One of the two patients who showed normal CPR figure at the time of hospitalization, or 50 percent, maintained the normal range.
The results confirmed the anti-inflammatory effects of Foistar, raising expectations to improve major symptoms of Covid-19, including fever and pneumonia, and prevent its aggravation, Daewoong said. The tablets also showed it is safe, as no patient showed adverse effects of hyperkalemia, known as the existing adverse reaction.
Daewoong expects to secure similar efficacy in phase 2 clinical trials. It also plans to develop Foistar tab into the nation’s first-and-primary oral drug to administer on Covid-19 patients, people with close contacts to patients, suspected patients, and self-quarantine patients.
“The results allow predicting the effect of controlling heat and inflammatory reactions when administering the drug to Covid-19 patients,” said Professor Choi Jae-phil of the Department of Infectious Diseases at the Seoul Medical Center, who led the team.
This study is significant because it is the first to confirm the anti-inflammatory effect on Covid-19 patients. Researchers will continue to contribute to the development of the treatment with further study, he added.
Daewoong Pharmaceutical CEO Jeon Seung-ho said, “Foistar is similar to Tamiflu tabs, which is safe and can be taken immediately. We will strive to secure clinical results within this year and provide them to patients from next January.”
The company plans to publish the result on medical journal websites.

