Lunit said its chest X-ray AI imaging analysis solution, Lunit INSIGHT CXR, will be covered by national health insurance in Korea from as early as March 2024 because it has been designated as an innovative medical technology by the Ministry of Health and Welfare through the "Integrated Review and Evaluation System for Innovative Medical Devices" notification.
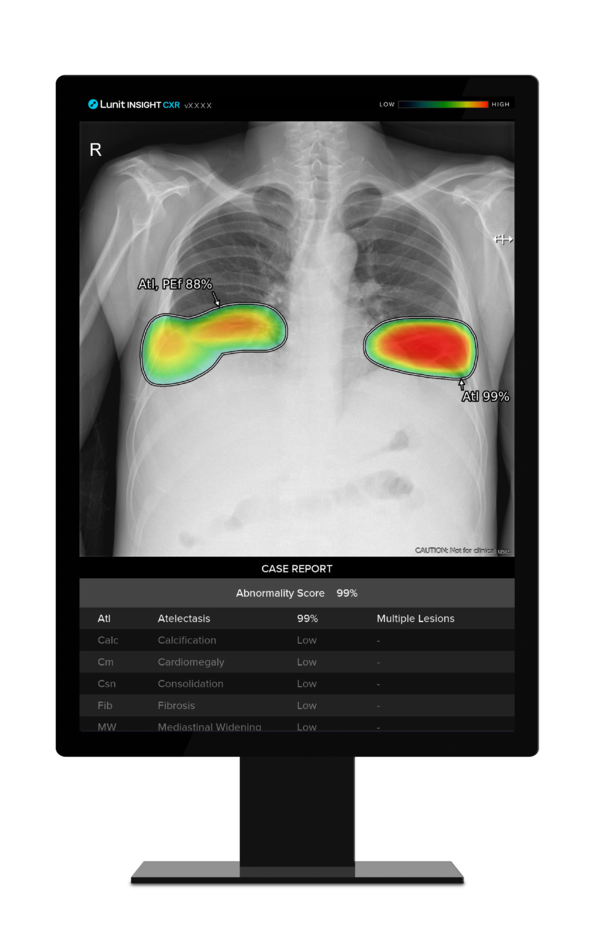
According to the notification results, Lunit INSIGHT CXR has been approved for clinical use as a safe and potentially innovative medical technology for three years, from March 1, 2024, to Feb. 28, 2027.
The innovative medical technology system is designed by the Ministry and the National Evidence-based Healthcare Collaborating Agency (NECA) to promote the use of promising future technologies.
When the potential value of the safety and efficacy of innovative medical technology is recognized, it can temporarily enter the medical market for validation and formal listing.
With this, Lunit can enter the selective benefit or non-benefit market for the next three years.
The company plans to actively pursue health insurance reimbursement for its AI medical devices as early as March next year. Additionally, within three years, Lunit intends to re-evaluate the new medical technology assessment and proceed with formal health insurance listing.
Through the integrated review and evaluation system for Lunit INSIGHT CXR, Lunit has completed all necessary steps, including reconfirming existing innovative medical device designation, confirmation of eligibility for new medical technology assessment application, and new approval of innovative medical technology.
Only the last step remains, which is the application process for temporary reimbursement listing with the Health Insurance Review and Assessment Service.
"With the approval of the innovative medical technology of Lunit INSIGHT CXR, we expect an expansion of the user base for our chest X-ray AI product," Lunit CEO Suh Beom-seok (Brandon Suh) said. "Once the integrated review and evaluation is complete, we expect to gain business momentum by introducing products eligible for reimbursement into the medical field as early as the first quarter of next year."
Suh stressed that Lunit is also undergoing the process of applying for the "Integrated Review and Evaluation System for Innovative Medical Devices" for its mammography AI imaging analysis solution, Lunit INSIGHT MMG, this month to pursue health insurance reimbursement.
Related articles
- Lunit to deploy AI chest screening to 5 Saudi hospitals, expanding presence in Middle East
- Lunit's AI-powered 3D breast tomosynthesis solution earns FDA clearance
- Lunit, CancerX leverage AI-powered solutions to reduce financial toxicity for cancer
- Lunit to provide AI-powered chest screening solution to the Philippines
- Lunit agrees with MD Anderson on AI-based research of immunotherapies’ efficacy
- Korean medical AI imaging companies to showcase products at RSNA 2023
- Lunit publishes 'Lunit Scope IO' study in Nature’s sister publication
- Lunit to acquire Volpara Health for $193 million to expand AI cancer screening in US
- Lunit inks $1.86 million deal with Samsung to supply AI solutions for chest X-ray reading

